1.0 Introduction
Cancer is one of the leading causes of death in developed countries, responsible for about 25% of all deaths. On a yearly basis, 0.5% of the population is diagnosed with cancer. Medically termed as a malignant neoplasm, cancer is an uncontrolled growth of abnormal cells in the body. This uncontrolled growth of cells can be attributed to mutations in the signals that regulate the cell cycle of growth and division (Priya, 2012). Most cells remain in interphase, the period between cell divisions, during the cell cycle. The initial stage of the interphase, G1 (for the first gap) involving rapid growth, metabolic activity, and synthesis of RNA. Next, is the S phase (for DNA synthesis), where the cell continues to grow, and DNA gets replicated. In G2 (for the second gap), the cell continues to grow and prepares for cell division. Next is Cell division (mitosis) called as M phase (Rolz-Cruz and Kim, 2008).
Cells that do not divide for long periods do not replicate their DNA and are in G0. In normal cells, tumor suppressor genes act as braking signals during G1 to stop or slow the cell cycle before the S phase. DNA repair genes are active throughout the cell cycle, particularly during G2 after DNA replication and before the chromosomes prepare for mitosis (Preety, 2012). Cancer cells overcome the normal cell signaling process and become immortal.
Apart from cell proliferation becoming uncontrollable, cells becoming resistant to apoptosis (programmed cell death) is another reason behind cancerous cells. In regard to mutations in tumor suppressors or activation of proto-oncogenes to oncogenes, the cell cycle gets disrupted, and the cells divide uncontrollably and grow into a lump called a tumor. (Tatarkova et al., 2012). In a benign tumor, the cells do not spread to other parts of the body and it is not called cancer. However, the cells may carry on growing at the original site and may cause a problem by pressing on other parts of the body. In a malignant tumor, the cells are able to spread to other parts of the body (Reeder and Vogel, 2007). Cancer will begin to grow in one part of the body. This is called primary cancer. If the cancer is not treated it may spread. If it spreads and grows in another part of the body, it is called secondary or metastatic cancer (Vlastos et al., 2004)
Attempts to cure or palliate cancer employ 3 principal methods: surgery, radiotherapy, and chemotherapy. Differing from the operation and radiotherapy that emphasize the treatment of local tissues, chemotherapy is concerned with that of the whole body. Chemotherapy is the use of drugs to inhibit or kill proliferating cancer cells while leaving host cells unharmed, or at least recoverable (Soares and Johnson, 2007). The drugs are carried in the blood so they can reach cancer cells in most parts of the body. Chemotherapy drugs are usually given into a vein or by mouth. Sometimes they are given by injection under the skin, into the muscle, into the fluid around the spine or into a body cavity such as the bladder, or by the use of creams may for some skin cancers (Vitiello et al., 2007).
Chemotherapy drugs can’t tell the difference between reproducing cells of normal tissues (like those that are replacing worn out normal cells) and cancer cells. This means normal cells are damaged along with the cancer cells, and this causes side effects. For this reason, the range and effectiveness of chemotherapy become restricted. Each time chemotherapy is given, it involves trying to find a balance between destroying the cancer cells (in order to cure or control the disease) and sparing the normal cells to lessen unwanted side effects (Rolz-Cruz and Kim, 2008; Smith and Dowsett, 2003). Determination of cancer type and cancer stage is a very important phenomenon for the success of chemotherapy (Kovacic, 2007). The growing capability of cancer cells to resist the chemotherapeutic drug is one of the main drawbacks of conventional chemotherapy (Johnstone et al., 2000). Over-dosage in chemotherapy can produce many harmful effects in the patients.
Side-effects occur mostly when the chemotherapy damages the healthy cells that maintain the body’s function and appearance. Different side effects arise depending on the nature of the drug. For example, the Alkalyting agents and anti-metabolites directly damage DNA to prevent the cancer cells from reproducing; thus they can cause long- term damage to the bone marrow, which can eventually lead to acute leukemia. Similarly, Anthracyclines can permanently damage the heart if given in high doses for a long time. Other side effect includes symptoms of allergic reaction like fast heartbeat, itching or hives, swelling in the face or hands, swelling or tingling in the mouth or throat, chest tightness, and wheezing. Immune-suppression, Bone marrow suppression leading to anemia and thrombocytopenia. Another serious problem of immune- compromised patients, caecitisis-infection of the gut- includes diarrhea, nausea, vomiting, fever, and distended abdomen, etc (Keidan et al., 2009). Infertility arises in both female and male patients by some gonad toxic chemotherapeutic agents may also occur (Brydøy et al., 2001). This review update, therefore, focused on problems and prospects associated with chemotherapy, addressing the mechanism involved in cancer formation, groups of chemotherapeutic agents as well as methods of chemotherapeutic administration.
2.0 Cancer
Medically termed as malignant neoplasm, cancer is an uncontrolled growth of abnormal cells in the body. This uncontrolled growth of cells can be attributed to mutations in the signals that regulate the cell cycle of growth and division (Priya, 2012). The cell cycle has 5 phases. Since cell reproduction happens over and over, the cell cycle is shown as a circle. All the phases lead back to the resting phase (G0), which is the starting point. When a cell goes through the cell cycle, it reproduces 2 new identical cells. Each of the 2 cells made from the first cell can go through this cell cycle again when new cells are needed.
G0 phase (resting stage): The cell has not yet started to divide. Cells spend much of their lives in this phase. Depending on the type of cell, G0 can last from a few hours to a few years. When the cell gets a signal to reproduce, it moves into the G1 phase.
G1 phase: The cell starts making more proteins and growing larger, so the new cells will be of normal size. This phase lasts about 18 to 30 hours.
S phase: The chromosomes containing the genetic code (DNA) are copied so that both of the new cells formed will have matching strands of DNA. This phase lasts about 18 to 20 hours.
G2 phase: The cell checks the DNA and gets ready to start splitting into 2 cells. This phase lasts from 2 to 10 hours.
M phase (mitosis): The cell actually splits into 2 new cells. This phase lasts only 30 to 60 minutes.
In normal cells, tumor suppressor genes act as braking signals during G1 to stop or slow the cell cycle before S phase. DNA repair genes are active throughout the cell cycle, particularly during G2 after DNA replication and before the chromosomes prepare for mitosis (Preety, 2012).
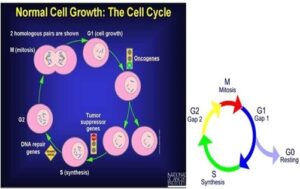
Figure 1: a. The cell cycle; b. Cell cycle phase Source: (Preety, 2012).
Cancer cells overcome the normal cell signaling process and become immortal. Apart from cell proliferation becoming uncontrollable, cells becoming resistant to apoptosis (programmed cell death) is another reason behind cancerous cells. In regards to mutations in tumor suppressors or activation of proto-oncogenes to oncogenes, the cell cycle gets disrupted, and the cells divide uncontrollably. Normal cells remain in the area where they belong and do not spread to other parts of the body. Cancer cells disregard this principle and spread through the body in a process called metastasis. These include direct invasion and destruction of the organ of origin, or spread through the lymphatic system or bloodstream to distant organs. The immune system consists of a group of cells called white blood cells that recognize and destroy “foreign” material in the body such as bacteria, viruses, and unfamiliar or abnormal cells.
Cancer cells manage to slip through this detection system without triggering the immune system to start fighting, either at the primary cancer site, in the blood vessels, or at the site of the distant spread (Priya, 2012)
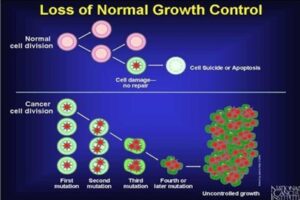
Fig 2: Cell division: normal cell vs. cancerous cell Source: (Preety, 2012).
2.1 Cancer Stem Cell (Csc)
A recent American Association for Cancer Research (AACR) workshop defined CSC as a malignant cancer cell with a stem cell phenotype (Clarke et al., 2006). Whilst the CSC hypothesis does not specifically address the mechanisms of malignant transformation, it has been suggested that CSCs are the malignant counterparts of normal adult tissue SCs which, due to dysregulated signaling pathways, are unable to maintain stem cell homeostasis. As well as the normal Scs, also CSCs are thought to reside at the top of the lineage hierarchy and give rise to differentiated cells, which themselves have no potential for self-renewal, and therefore do not contribute significantly to tumor growth. Due to their long life, SCs remain in a tissue for longer periods compared to their differentiated progeny, thereby making them more likely to acquire transforming mutations. Additionally, it is generally accepted that SCs are more resistant to apoptosis and DNA damage and they are therefore more likely to survive to any insults (Croker and, Allan, 2008).
Whilst being quiescent in normal tissue, SCs are able to maintain their pool by undergoing asymmetric cell division during biological processes such as the occurrence of tissue damage. During this process, a SC divides asymmetrically to generate an identical daughter cell that is committed to differentiation. It has been suggested that in this way CSCs generate the different cell types within a tumor, leading to tumor self renew as well. Specific signaling pathways are involved in embryogenesis processes, leading to the development of various organs. We are talking about several key pathways, such as sonic Hedgehog, Notch, PTEN, BMI-1, WNT, and p53. During the development of cancer an alteration of these pathways occurs and this event could lead to dysregulation of SC self- renewal and contribute to tumor proliferation (Molofsky et al., 2004). The SC pool is also tightly regulated by signaling pathways from the microenvironment of the SC niche, and several of these pathways, including Hedgehog and WNT, have been implicated in carcinogenesis (Korkaya et al., 2009).
This may have very important implications in therapeutic interventions, including explanation for the development of chemoresistance. A role for CSCs in propagating and maintaining metastases has been proposed (Miki et al., 2007). The different hypothesis that the dedifferentiation of mature cells to a more pluripotent state could be a potential mechanism for the development of SC-like features by cancer cells, cannot be dismissed. In 1997 Bonnet and Dick first isolated the CSCs in leukemic cells expressing SC marker CD34 and afterwards, also, in other solid tumors (Bonnet and Dick, 1997). Classically, SCs are defined by their two main characteristics: self-renewal and pluripotency (Quintana et al., 2008). Experiments performed on human acute myeloid leukemia and solid tumors show that CSC have three functional characteristics: transplantability, tumorigenic potential to form tumors when injected into nude mice; distinct surface markers; ability to recreate the full phenotypic heterogeneity of the parent tumor (Clarke, 2007). In characterizing normal and CSC s the problem is that these cellular populations are rare and the absence of specific cell surface markers represents a challenge to isolate and identify pure SC populations (Hill, 2006)
2.2 Cancer Cell Metabolism
Glucose is one of the most important energy sources of proliferating cells. In the first step of energy production, glucose is metabolized by glycolysis to pyruvate. Under aerobic conditions, pyruvate is oxidatively decarboxylated by the pyruvate decarboxylase complex inside the mitochondria to form acetyl Coenzyme A (acetyl-CoA). Acetyl-CoA is then completely oxidized to CO2 by the tricarboxylic acid (TCA) cycle. This process provides NADH, which is oxidized during mitochondrial respiration to generate high amounts of ATP through oxidative phosphorylation (OXPHOS). Under anaerobic conditions, pyruvate can be reduced (‘fermented’) to lactate by lactate dehydrogenase (LDH). This process of glucose fermentation is less efficient in generating ATP than the TCA cycle (Lunt et al., 2011). In the early 1920s, Warburg first noticed that cancer cells consume more glucose than normal cells leading to increased production of lactic acid even in the presence of sufficient amounts of oxygen (designated as ‘aerobic glycolysis’). This phenomenon is known as the “Warburg effect” (Vander et al., 2009).
Higher glucose uptake in tumors is used diagnostically for cancer detection by monitoring the incorporation of [18F]- labeled deoxyglucose (FDG) in FDG-positron emission tomography. So far, underlying mechanisms for the altered metabolic program in cancer cells are still not completely elucidated. Changes in the tumor’s microenvironment, such as limited availability of oxygen and nutrients, may influence the metabolic switch from OXPHOS to aerobic glycolysis (Vander, 2011). In addition, various genetic alterations in pathways regulating cell metabolism have been identified during the past years. These include for example the PI3K (phosphatidylinositol-3-kinase), HIF (hypoxia- inducible factor), p53, c-Myc, Ras, AMPK (AMP- activated protein kinase) and LKB1 (liver kinase B1) pathways, as well as overexpression of glucose transporters (Glut), hexokinase (HK) and other key enzymes involved in glycolysis. Stimulated by the c-Myc oncogene, cancer cells exceedingly utilize glutamine as a carbon and nitrogen source as well as for the generation of ATP (Lunt et al., 2011).
Cancer cells adapt to the metabolic requirements of tumor growth by alterations in the activity of pyruvate kinase (PK) (Mazurek et al., 2005). PK catalyses the rate limiting, ATP- generating step of glycolysis, in which phosphoenol- pyruvate (PEP) is converted to pyruvate. Surprisingly, cancer cells express high levels of the less efficient embryonic M2 isoform of PK (PKM2), resulting in the inhibition of glycolysis and reduced production of ATP. The advantage for cancer cells lies in the fact that intermediates of glycolysis accumulate and are available for alternative metabolic pathways. Rapidly dividing cells need biosynthetic intermediates for cell duplication during proliferation.
Channeling glucose to aerobic glycolysis instead of OXPHOS enables the cell to export acetyl-CoA to the cytosol in form of citrate for the construction of fatty acids, to supply glycolytic intermediates for nonessential amino acid production, and to provide ribose-5-phosphate from the pentose phosphate pathway (PPP) for nucleotide synthesis. Additionally, the pentose phosphate pathway promotes the production of NADPH as an important cofactor that supplies reducing equivalents for many enzymatic reactions (Cairns et al., 2011). NADPH is also a key antioxidant required to control levels of reactive oxygen species (ROS) produced exceedingly during rapid cell proliferation (Cairns et al., 2011).
Therefore, the altered cancer cell metabolism is likely to constitute a growth advantage to rapidly proliferating cancer cells (Vander et al., 2009).
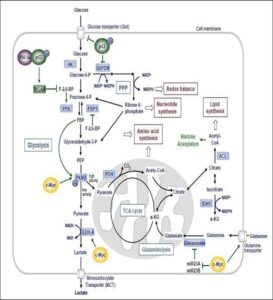
Figure 3. Interconnection of glycolysis, oxidative phosphorylation via the tricarboxylic (TCA) cycle, the pentose phosphate pathway (PPP) and glutaminolysis in proliferating cancer cells. Sources: (Cioce and Blandino, 2011).
2.3 Epigenetic Regulation
The term “epigenetics” refers to modifications in gene expression caused by heritable, but potentially reversible, alterations in chromatin structure and/or DNA methylation without changes in the DNA sequence. Epigenetic mechanisms play an important role for diverse cellular processes, for example during development, tissue specific gene expression and memory formation (Stilling and Fischer, 2011). However, epigenetic mechanisms are also involved in the development of age- and lifestyle-related diseases such as metabolic syndrome, cancer and Alzheimer’s disease (Bruce and, Cagampang, 2011). Best investigated epigenetic modifications comprise of DNA methylation, posttranslational histone modifications including acetylation, methylation, phosphorylation, ubiquitination and sumoylation, and regulation of gene expression by noncoding (nc) RNA including microRNA (Jaenisch and Bird, 2003). These epigenetic modifications can be inherited to daughter cells. Thus epigenetic modifications have the potential to correct altered gene expression patterns that have been established as a consequence of environmental stimuli (e.g. nutrition, chemical exposure, radiation etc.) or as a result of signals in a cells microenvironment and therefore could act as a ‘memory’ for gene expression patterns (Herceg et al., 2011).
3.0 Cancerchemotherapy
3.1 History of cancer chemotherapy
The first practical anticancer drugs were discovered accidentally. One such discovery was an outcome of war, stemming from the finding that sulfur mustard gas, used as a toxic vesicant in the First World War, caused myelosuppression. Although gas warfare was not employed in the Second World War, a considerable stock of mustard gas canisters was maintained in the Mediterranean area. An accident in the Italian port of Bari, involving leakage of one of these canisters, rekindled interest in the myelosuppressive effect of nitrogen mustard, leading to clinical trials in lymphoma patients (Kohn, 1996).
The identification of vitamins as small low-molecular weight enzyme cofactors was an important biochemical achievement in the early part of the 20th century. The structural elucidation and crystallization of folic acid in 1946 led, as with other isolated vitamins, to studies on its effect on the course of a number of diseases. Unexpectedly, administration to leukemia patients of folic acid and its glutamylated derivatives resulted in an increase in tumor growth. While the use of low-folate diets in the management of leukemia was investigated, the development of the folic acid analogue aminopterin provided a significant advance in the management of childhood acute leukemia (Bertino, 1979).
The link between these two disparate types of drugs and their biological activity was found to be related to their damaging effect on DNA. Although Friedrich Miescher had characterized DNA as a substance in 1862, the informational complexity and significance to life of DNA was not appreciated until the 1940s. The elucidation in 1953 by James Watson and Francis Crick of the double- helical structure of DNA had a singular impact on strategies of anticancer drug development. The cancer chemotherapeutic agent nitrogen mustard was found to react chemically with DNA (Kohn et al., 1966). Studies on aminopterin indicated that it interrupted DNA biosynthesis and in so doing caused DNA damage. The next two decades brought a massive development of new drugs that affected the integrity of the cell’s genetic material, with approximately one new drug entering widespread clinical use every 2 years. Many of these drugs, which revolutionized the treatment of many types of cancer, are shown in Figure 4.
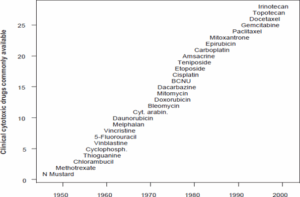
Figure 4. Chronology for the development of some of the anticancer drugs currently in use today. The abbreviations are N mustard (nitrogen mustard), cyt. arabin. (cytosine arabinoside), and BCNU (bischloroethylnitrosourea).
3.2 Classification of Chemotherapy Drugs
Chemotherapy drugs can be divided into several groups based on factors such as thie mechanism of action, their chemical structure, and their relationship to another drug. Some drugs act in more than one way, and may belong to more than one group. Knowing how the drug works is important in predicting side effects (Missailidis, 2008).
3.2.1 Alkylating agents
Alkylating agents directly bind to DNA, thereby inhibiting DNA replication and initiating cell death. These drugs work in all phases of the cell cycle and are used to treat many different cancers, including leukemia, lymphoma, Hodgkin disease, multiple myeloma, and sarcoma, as well as cancers of the lung, breast, and ovary (Pereg et al., 2008). Because these drugs damage DNA, they can cause long-term damage to the bone marrow. In rare cases, this can lead to acute leukemia. The risk of leukemia from alkylating agents is “dose-dependent,” meaning that the risk is small with lower doses, but goes up as the total amount of the drug used gets higher.
The risk of leukemia after getting alkylating agents is highest about 5 to 10 years after treatment. Alkylating agents are divided into different classes, including: Nitrogen mustards: such as mechlorethamine (nitrogen mustard), chlorambucil, cyclophosphamide (Cytoxan®), ifosfamide, and melphalan, Nitrosoureas such as streptozocin, carmustine (BCNU), and lomustine, Alkyl sulfonates such as busulfan, Triazines such as dacarbazine (DTIC) and temozolomide (Temodar), Ethylenimines such as thiotepa and altretamine (hexamethylmelamine) (Pereg et al., 2008)
3.2.2 Platinum compounds
Platinum compounds form DNA adducts that result in DNA crosslinking. DNA crosslinks inhibit replication, transcription, and other nuclear functions. The combination of these events arrests cell proliferation and ultimately tumour growth. Cisplatin and carboplatin are among the most commonly used platinum compounds (Koren et al., 2005).
3.2.3 Antimetabolites
Antimetabolites are small compounds that act as false substrates during DNA or RNA synthesis. Hey interfere with DNA and RNA growth by substituting for the normalbuilding blocks of RNA and DNA. These agents damage cells during the S phase, when the cell’s chromosomes are being copied. They are commonly used to treat leukemias, cancers of the breast, ovary, and the intestinal tract, as well as other types of cancer. 5-fluorouracil (5-FU),6-mercaptopurine (6- MP), Capecitabine, Cytarabine, Floxuridine,Fludarabine,Gemcitabine,hydroxyurea,Methot rexate and Pemetrexed are among those commonly used antimetabolites (Azim and Peccatori, 2011).
3.2.4 Anti-tumor antibiotics
Microorganisms produce these cytotoxic agents that interact directly with DNA resulting in anti-cancer activity. The manner in which antibiotics interact with DNA differs considerably between agents. These drugs are not like the antibiotics used to treat infections. They work by altering the DNA inside cancer cells to keep them from growing and multiplying (Cardonick et al., 2010). Anthracyclines are anti-tumor antibiotics that interfere with enzymes involved in DNA replication. These drugs work in all phases of the cell cycle. They are widely used for a variety of cancers. Examples of anthracyclines include: Daunorubicin, Doxorubicin, Epirubicin and Idarubicin. A major concern when giving these drugs is that they can permanently damage the heart if given in high doses. For this reason, lifetime dose limits are often placed on these drugs. Other anti-tumor antibiotics that are not anthracyclines include: Actinomycin-D, Bleomycin, Mitomycin-C and Mitoxantrone (also acts as a topoisomerase II inhibitor) (Van Calsteren et al., 2010).
3.2.5 Topoisomerase inhibitors
These drugs interfere with enzymes called topoisomerases, which help separate the strands of DNA so they can be copied during the S phase. Topoisomerase inhibitors are used to treat certain leukemias, as well as lung, ovarian, gastrointestinal, and other cancers. Topoisomerase inhibitors are grouped according to which type of enzyme they affect: Topoisomerase I inhibitors include: Topotecan and Irinotecan (CPT-11). While Topoisomerase II inhibitors include: Etoposide (VP-16), Teniposide and Mitoxantrone (also acts as an anti-tumor antibiotic). Topoisomerase II inhibitors can increase the risk of a second cancer – acute myelogenous leukemia (AML) – as early as 2 to 3 years afterthe drug is given (Missailidis, 2008).
3.2.6 Mitotic inhibitors
Mitotic inhibitors are often plant alkaloids and other compounds derived from natural products. They work by stopping mitosis in the M phase of the cell cycle but can damage cells in all phases by keeping enzymes from making proteins needed for cell reproduction. Examples of mitotic inhibitors include: Estramustine, Taxanes such as paclitaxeland docetaxel, Epothilones such as ixabepilone, Vinca alkaloids such as vinblastine, vincristine, and vinorelbine, They are used to treat many different types of cancer including breast, lung, myelomas, lymphomas, and leukemias. These drugs may cause nerve damage, which canlimit the amount that can be given (Pereg et al., 2008).
3.2.7 Corticosteroid
Corticosteroids, often simply called steroids, are natural hormones and hormone-like drugs that are useful in the treatment of many types of cancer, as well as other illnesses. When these drugs are used as part of cancer treatment, they are considered chemotherapy drugs. Examples of corticosteroids include: Prednisone, Methylprednisolone and Dexamethasone. Steroids are also commonly used to help prevent nausea and vomiting caused by chemotherapy. They are used before chemotherapy to help prevent severe allergic reactions, too (Koren et al., 2005).
3.2.8 Other chemotherapy drugs
Some chemotherapy drugs act in slightly different ways and do not fit well into any of the other categories. Examples include drugs like L-asparaginase, which is an enzyme, and the proteosome inhibitor bortezomib.
3.3 Effects of Vitamins On Chemotherapy
Many people want to take an active role in improving their overall health. They want to help their body’s natural defenses fight the cancer and speed up their recovery from chemo. Because most people think of vitamins as a safe way to improve health, it’s not surprising that many people with cancer take high doses of one or more vitamins. But few know that some vitamins might make their chemo less effective (Azim and Peccatori, 2011). Certain vitamins, such as A, E, and C act as antioxidants. This means that they can prevent formation of ions (free radicals) that damage DNA. This damage is thought to have an important role in causing cancer. But some chemotherapy drugs (as well as radiation treatments) work by producing these same types of free radical ions. These ions severely damage the DNA of cancer cells so the cells are unable to grow and reproduce. Some scientists believe that taking high doses of antioxidants during treatment may make chemo or radiation less effective (Cardonick et al., 2010; Van Calsteren et al., 2010).
3.4 Methods of Chemotherapy Administration
In most cases, chemo drugs are put right into the bloodstream or taken as pills. They then travel throughout the body to kill cancer cells. Sometimes there’s a need to get high doses of chemo to a specific area of the body. Regional chemotherapy directs the anti-cancer drugs into the part of the body where the cancer is. The purpose is to get more of the drug to the cancer, while trying to limit effects on the whole body. Side effects will often still happen because the drugs can be partly absorbed into the bloodstream and travel throughout the body (Cardonick et al., 2010). Examples of regional chemo include drugs given into these parts of the body
3.4.1 Intra-arterial chemo
An intra-arterial infusion allows a chemo drug to be given right to the tumor through a small, flexible tube (catheter) that’s put in the main artery that supplies blood to the tumor. This method is used to treat disease in an organ such as the liver (this is called isolated hepatic perfusion), or to treat an extremity such as the leg (called isolated limb perfusion).
The goal is to concentrate the drug in the area of the tumor and decrease systemic side effects. The catheter is attached to an implanted or portable pump. Although this approach sounds like a good idea, most studies have not found it to be as useful as expected. This approach is being studied for many types of cancer in clinical trials. Except for clinical trials, it’s rarely available outside of specialized cancer centers (Missailidis, 2008).
3.4.2 Intracavitarychemo
Intracavitary is a broad term used to describe chemo given right into a body cavity. The chemo drug is given through a catheter that’s put into one of the areas as described below.
- Intravesicalchemotherapy is often used for early stage bladder The chemo is usually given weekly for 4 to 12 weeks. For each treatment, a soft, flexible tube (called a urinary catheter) is put into the bladder to give the drug. The drug is kept in the bladder for about 2 hours and then drained. The catheter is taken out after each treatment (Van Calsteren et al., 2010).
- Intrapleuralchemotherapy is not used very often but may be helpful for some people with mesothelioma (cancer that develops in the lining of the lung), and those with lung or breast cancers that have spread to the pleura (the membrane around the lungs and lining the chest cavity). Intrapleural chemotherapy is given through soft, flexible tubes called chest catheters. These catheters can be used to give drugs and to drain fluid that can build up in the pleural space when cancer has spread to that area (NCI, 2015).
- Intraperitoneal chemotherapy has become one of the standard treatments for certain stages of ovarian It may also be used to treat some colon cancers that come back after treatment, as well as mesotheliomas and cancers of the appendix, liver, or stomach that have spread throughout the belly (abdomen). Intraperitoneal chemo is given through a Tenckhoff catheter (a soft tube specially designed for removing or adding large amounts of fluid from or into the abdominal cavity) or through an implanted port (a small drum-like device) attached to a catheter. Chemo injected into the port travels through the catheter into the abdominal cavity where it’s absorbed into the affected area before entering the bloodstream. This approach can work very well, but it can also have more severe side effects than chemo put into the bloodstream (IV chemo). The higher doses that are used, along with more gradual absorption of the drug into the body, may be part of why the side effects may be worse (NCI, 2015).
- Intrathecal chemotherapy is given right into the fluid surrounding and cushioning the brain and spinal cord(called the cerebrospinal fluid or CSF) to reach cancer cells in the fluid and the central nervous system (brain and spinal cord). Most chemo drugs that are put into the bloodstream are unable to cross the barrier between the bloodstream and the central nervous system, called the blood-brain barrier. Intrathecal chemotherapy gets the drug directly to the central nervous system. Intrathecal chemotherapy is given in 1 of 2 ways:
The chemo can be given by a lumbar puncture (spinal tap) done daily or weekly. This is when a thin needle is placed between the bones of the lower spine and into the space through which the CSF flows around the spinal cord (NCI, 2015).
A special device called an Ommaya reservoir can be used. It’sa small, drum-like port that’s placed under the skin of the skull. An attached catheter goes through the skull into a ventricle (a space inside the brain filled with CSF). A special needle is put through the skin and into the port to give the chemo (Van Calsteren et al., 2010).
Chemo is given this way when it’s needed to treat cancer cells that have entered the central nervous system. This is seen most commonly in leukemias, but also may happen with some lymphomas and advanced solid tumors like breast and lung cancers. Intrathecal chemotherapy does nothelp when tumors have already started growing in the brain or spinal cord (Cardonick et al., 2010).
3.4.3 Intralesional chemo
Intralesional chemo refers to the drug being injected directly into the cancerous tumor. It may be used for tumorsthat are in or under the skin, and rarely for tumors that are on an organ inside the body. It’s only possible when the tumor can be safely reached by a needle, and is most often used when surgery isn’t an option.
3.4.4 Topical chemo
In this use, chemo is put on the skin in the form of a cream or lotion. Most often, it’s used to treat basal cell or squamous cell skin cancers. It’s also used to treat pre- cancerous growths on the skin. The patient or a family member usually puts on the chemo cream. It’s important to understand the schedule, know exactly how to use these potent drugs, and know what kinds of precautions to use (NCI, 2015).
3.5 Cancer and Pregnancy
The diagnosis of cancer during pregnancy poses difficult dilemmas for the pregnant patient, her family, and the medical team. Cancer in pregnancy is rare, complicating up to 0.02% to 0.1% of pregnancies annually (NCI, 2015). The cancers most commonly diagnosed in pregnancy are breast cancer, cervical cancer, thyroid cancer, Hodgkin’s lymphoma, and non Hodgkin’s lymphoma. The main challenge in managing cancer during pregnancy is treating the patient with the optimal anti-cancer regimen without harming the developing fetus. Critically, for the best chance at survival for the mother, chemotherapy cannot always be postponed until the end of the pregnancy, and no current regimen has been confirmed (by studies with sufficient statistical power) safe for use during gestation. Because of their relatively low molecular weight, most cytotoxic agents cross the placenta and reach the fetus (Cardonick and Iacobucci, 2004).
The pharmacology of various anti-cancer drugs may be altered by the normal physiological changes that occur during pregnancy, such as increased plasma volume, enhanced renal and hepatic elimination, and decreased albumin concentration. Dosing similar to that of nonpregnant women of the same weight may lead to undertreatment of pregnant women with cancer. However, it is still not clear whether pregnant women should be treated with different doses of chemotherapy, and no studies have addressed the effectiveness of treatment regimens in pregnancy (Van Calsteren et al., 2010).
Chemotherapy during the first trimester may increase the risk of spontaneous abortions, fetal death, and major congenital malformations. The teratogenic effects depend on the dosage, time of administration, and cumulative exposure to the chemotherapeutic agent. Fetal malformations reflect the gestational age at exposure, and the most vulnerable period is during weeks 2 to 8, when organogenesis occurs. The eyes, ears, teeth-palate, genitalia, hematopoietic system, and CNS remain vulnerable to chemotherapy beyond organogenesis (Cardonick and Iacobucci, 2004)
The administration of chemotherapy during the second andthird trimesters has not been associated with major congenital malformations but may increase the risk for intrauterine growth restriction (IUGR), low birth weight, and stillbirth. A review of 376 cases of fetuses exposed to chemotherapy in utero, most after organogenesis, demonstrated 5% fetal death rate and 1% neonatal death rate. Other complications included preterm delivery (5%), IUGR (7%), and transient myelosuppression (4%) (Van Calsteren et al., 2010).
4.0 Problems of Chemotherapy: Side-Effects
Chemotherapeutic drugs are designed on the basis of their killing ability of cells that grow faster, the main characteristic feature of cancer cells. However, chemotherapeutic drugs also kill normal cells that divide; e.g. bone marrow cells, gut cells, etc. For this reason, the range and effectiveness of chemotherapy becomes restricted.
The growing capability of cancer cells to resist the chemotherapeutic drug is one of the main drawbacks of conventional chemotherapy. In drug-resistant cancer cells, expression of surface small pump like p- glycoprotein and intercellular antioxidant efflux prevent the chemotherapeutic agents from entering into the cells (Johnstone et al., 2000). Many critical molecular types of machinery are involved in chemotherapeutic drugs and tumor cell interactions. Genotoxic drugs used in chemotherapy may induce alteration of gene expressions causing aggravation of DNA damage, resulting in cell death. But sometimes, structural changes in protein induced by genetic mutation prevent binding of drug with target protein, which in turn can resist the chemotherapeutic effect (Luqmani, 2005). Acquired capabilities of cancer cellsto produce gene amplification and alteration of gene expressions by mutation on coding genes for apoptosis- inducing proteins play an important role in generating resistance against chemotherapeutic drugs. Defect in apoptotic pathway by genetic alteration of normal cells that involve in tumor genesis can also resist the chemotherapeutic drugs which kill the cancer cells by apoptosis. Thus, mutation in genes involving development of cancer cell can prevent apoptosis and also play important role in drug resistance.
Over-dosage in chemotherapy can produce much harmful effects in the patients. Therefore, much care has to be taken to determine the effective dosage. Several factors like body surface area (BSA) formula, mathematical calculation on the basis of the patients’ weight and height had been approved in early 1950s for calculating individual chemotherapeutic dosage. The effectiveness of chemotherapeutic drug varies with concentration of the drug in the patient’s bloodstream which is subjected by multiple factors, including metabolism, cancer state, drug- target interactions, and patients’ physiological and genetically state ( Kaestner and Sewell, 2007). Now-a-days researchers are trying to establish a formula by which individual patient can achieve optimal systemic drug exposure which can maximize the drug efficacy and minimize cytotoxic effects.
Determination of cancer type and cancer stage is a very important phenomenon for success of chemotherapy. In certain cancer type where drug delivers through circulatory system, chemotherapy fails because of improper blood vessel in tumor cells (Minchinton and Tannock, 2006). Patients with late stage cancer may not fully cure and face greater risk of early death because they may not tolerate chemotherapeutic side-effects. Thus, side-effects are one of the major and leading problems of chemotherapy and often limit its use (Kovacic, 2007).
Side-effects occur mostly when the chemotherapy damages the healthy cells that maintain the body’s function and appearance. Different side-effects arise depending on the nature of the drug. As for example, the Alkalyting agents and anti-metabolites directly damage DNA to prevent the cancer cells from reproducing; thus they can cause long- term damage to the bone marrow, which can eventually lead to acute leukemia. Similarly, Anthracyclines are anti- tumor antibiotics that interfere with enzymes involved in DNA replication. These drugs work in all phases of the cell cycle. A major consideration when giving these drugs is that they can permanently damage the heart if given in high doses for a long time.
For this reason, lifetime dose limits are often put on place on these drugs. In the same way, some plant alkaloids and other compounds derived from natural products are often known to be mitotic inhibitors. They can stop mitosis or inhibit enzymes from making proteins needed for normal cell reproduction. These drugs are known for their potential to cause peripheral nerve damage, which can be a dose- limiting side effect. Likewise, topoisomerase inhibitors that cause DNA damage to cancer cells produce harmful side effects like symptoms of allergic reaction including fast heartbeat, itching or hives, swelling in the face or hands, swelling or tingling in the mouth or throat, chest tightness, and wheezing. Cytotoxic medicines are generally very powerful but often cause many unwanted side-effects. Since cytotoxic medicines mainly work by killing cells in their divisional stages, irrespective of whether they are normal or cancer cells, protection to normal cells is of prime importance while a patient is undergoing cancer therapy.
Immune-suppression during chemotherapy causes various side-effects also. Bone marrow suppression decreases the production of blood cells leading to anemia and thrombocytopenia. Another serious problem of immune compromised patients, caecitisis-infection of the gut- includes diarrhea, nausea, vomiting, fever and distended abdomen etc (Keidan et al., 2009). Prolonged autoinfectionmanifests as systemic disease conditions like sepsis (Boggio et al., 2000).
In early stage of chemotherapy mucositis, a painful inflammation and ulceration of mouth begins by damaging of normal cells lining the mouth (Sonis, 2004). Chemotherapeutic drugs target rapidly dividing cells like gastrointestinal cells resulting in nausea, vomiting, anorexia, diarrhea, abdominal cramps, and constipation etc. Gastrointestinal damage causes gastro paresis, decreased gut motility or delayed emptying of the stomach and small intestines leading to malnutrition and dehydration. Fatigue, one of the most frequent and long lasting side-effects of chemotherapy, is a very problematic issue and it imposes limitations on normal daily activities of about 70% patients (Berger et al., 2010). Every chemotherapeutic agent targets and damages fast dividing hair follicles resulting in vigorous hair loss leading to certain type of alopecia like alopecia totalis, telogen effluvium, or less often alopecia aerate (Chadha and Shenoi, 2003). Some Alkalyting chemotherapeutic agent constructs secondary neoplasia like acute myeloid leukemia which increases in frequency (Rüther et al., 2000). It has been observed that many chemotherapeutic patients lack their interest in sex (Stead, 2004).
Infertility arises in both female and male patients by some gonad toxic chemotherapeutic agents (Brydøy et al., 2001). During pregnancy chemotherapy makes congenital abnormalities including growth retardation, delayed mental development or other congenital disorders (Arnon et al., 2001). Patients may suffer from peripheral neuropathy, a damage or disease affecting nerves which produces symptoms like numbness, abnormalities in brain and spinal cord, tremor, and gait abnormality (del Pino, 2010). Many chemotherapeutic agents cause non-specific neuro-cognitive problems in which patients suffer from inability to concentrate. Some chemotherapeutic drugs rapidly break down and cancer cells in turn release chemicals from inside the cells producing high levels of uric acid, potassium, phosphate and calcium in the blood. One of the decisive problems during chemotherapy is damages inflicted on normal organs. Organ-specific toxicities arise by specific chemotherapeutic agents. Most chemotherapeutic drugs generate many free radicals producing Cytotoxicity, which in turn causes DNA damage in the cells leading finally to apoptosis. Cardio toxicity, hepatotoxicity, nephrotoxicity and ototoxicity, are some of the leading problems generating from the non- specific cytotoxic nature of these chemotherapeutic drugs (Shaikh, 2012). Chemotherapeutic drugs may also produce many other side-effects like dry skin and mouth, water retention, dental problem, digestive problem, emotional difficulties etc.
4.2 The Future of Primary Chemotherapy (pCR)
The increase in pCR rate seen in the docetaxel-containing arm of NSABP B-27 is an encouraging result. However, we do not know to what degree the improved pCR rate brought about by the addition of docetaxel will translate into an improvement in overall survival. But we do know that this systemic chemotherapy still fails to induce a pCR in three- fourths of treated patients. This suggests that we have a long way to go, therapeutically. What are reasonable future directions for primary therapy trials? Some directions will necessarily involve chemotherapeutic approaches.
The recent presentation of C9741, an intergroup trial testing the dose-density hypothesis, suggests that the use of granulocyte- colony-stimulating factor to narrow the interval between treatments may result in improved relapse-free and overall survival in the adjuvant setting. This approach certainly might be used in a primary chemotherapy setting, shortening the time before definitive surgery and perhaps increasing operability. Pilot dose- dense trials have been performed in the preoperative setting. Another approach would be to utilize docetaxel as a platform on to which other chemotherapeutic agents might be added. The obvious agent to consider in this context is capecitabine. In the setting of metastatic disease, the addition of capecitabine to docetaxel has been shown to improve overall survival; it is reasonable to expect that its addition in the adjuvant setting might improve the cure rate of patients with early disease. There is certain poignancy to this approach. In the United States during the 1990s, fluorouracil was widely dropped from adjuvant regimens in cooperative group trials, based on convenience factors rather any data suggesting inactivity in the adjuvant setting. Now we may see the return of the fluoropyrimidines in another guise.
The primary chemotherapy setting also offers nonchemotherapeutic possibilities. Preoperative trastuzumab–based regimens (in combination with chemotherapy) have already been piloted in the primary chemotherapy setting in patients with HER-2 positive disease, and are certainly worthy of consideration for confirmatory randomized trials. And while the vast majority of the preoperative therapy literature has examined the use of systemic chemotherapy, more recent trials have suggested a place for preoperative hormonal therapy for estrogen receptor– positive breast cancer (Miller et al., 2009). While we have no direct comparisons of preoperative hormonal therapy to preoperative chemotherapy, data from the adjuvant setting certainly suggests that this would be a reasonable approach for some patients (Burstein et al., 2001).
Finally, the preoperative therapy setting offers us some useful possibilities with regard to therapeutic targeting andtreatment individualization. The preoperative setting represents a potentially useful clinical laboratory for exploring surrogate markers of response. When one can obtain tissue before therapy, treat a patient, and then obtain residual tissue at the time of definitive surgery, the possibility exists for analysis of this tissue to evaluate molecular markers of response and resistance. As in every other aspect of oncology, novel technologies such as cDNA microarrays (“gene chips”) have the potential to revolutionize our understanding of response and resistance.
A cDNA microarray analysis of tumor samples obtained before primary chemotherapy has recently been presented, and suggests that this technology may soon be able to predict who will benefit from such therapy. NSABP B-27 has several important correlative trials that will be reported in coming years. There is no doubt that this important trial will continue to teach us new lessons about the biology and treatment of breast cancer (O’Shaughnessy et al., 2002).
4.3 Conclusion
Cancer, a dreadful disease, is one of the burning issues that need collective efforts to successfully combat and cure. Cancer is an uncontrolled growth of abnormal cells in the body. This uncontrolled growth of cells can be attributed tomutations in the signals that regulate the cell cycle of growth and division. In normal cells, tumor suppressor genes act as braking signals during the cell cycle. DNA repair genes are active throughout the cell cycle. Cancer cells overcome the normal cell signalling process and become immortal. Chemotherapy is one among many options like surgery, radiation therapy, targeted molecular therapy, etc., which is widely used to successfully treat certain types of cancer, but its use is restricted for the adverse side-effects of most of the chemotherapeutic drugs. The toxic by-products of some chemotherapeutic drugs are deposited mainly in the liver producing hepatotoxicity, leading to many side-effects like loss of appetite, nausea, vomiting, diarrhea, abdominal cramps and constipation, anemia, fatigue, hyperthermia, loss of hair, etc.
Conflict of Interest
The author declared no conflict of interest exist
Authors contributions
The work was conducted in collaboration of all authors. All authors read and approved the final version of the manuscript.
References
Ahmed, S., Stewart, J.H., Shen, P., Votanopoulos, K.I. and Levine, E.A. (2014). Outcomes with cytoreductive surgery and HIPEC for peritoneal metastasis. J Surgery Oncology. 110(5):575- 584.
Alberg, A.J. and Samet, J.M. (2003). Epidemiology of Lung Cancer. Chest. 123:21-49.
Albrand, G. and Terret, C. (2008) Early breast cancer in the elderly assessment and management considerations. Drugs Aging 25, 35-45.
Armstrong, D.K., Bundy, B. and Wenzel, L. (2006). Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34-43.
Arnon, J., Meirow, D., Lewis-Roness, H. and Ornoy, A.(2001). Genetic and teratogenic effects of cancer treatments on gametes and embryos. Hum Reprod Update. 7: 394-403.
Azim, H.A. and Peccatori, F.A. (2011). Managing cancer during pregnancy: what evidence do we have? Pol Arch Med Wewn. 121:29–34.
Berger AM, Abernethy AP, Atkinson A, Barsevick AM, Breitbart WS, Cella D, et al. Cancer-related fatigue. J Natl Compr Canc Netw. 2010; 8: 904-931.
Bertino, J. R. (1979). Nutrients, vitamins and minerals as therapy. Cancer43, 2137–2142
Boggio, L., Pooley, R., Roth, S.I. and Winter, J.N. (2000). Typhlitis complicating autologous blood stem cell transplantation for breast cancer. Bone Marrow Transplant. 2000; 25: 321-326.
Bonnet, D., Dick, J.E. (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature, 3:730–737.
Brocard ,J,. Kastner, P., Chambon, P. (1996). Two novel RXR alpha isoforms from mouse testis. Biochem Biophys Res Commun. . 229:211–218,
Bruce, K.D., Cagampang, F.R.(2011). Epigenetic priming of the metabolic syndrome. Toxicological Methods : 21: 353-361.
Brydøy, M., Fosså, S.D., Dahl, O. and Bjøro, T.(2007). Gonadal dysfunction and fertility problems in cancer survivors. Acta Oncology. 46: 480-489.
Cairns ,R.A., Harris, I.S., Mak, T.W.(2011). Regulation of cancer cell metabolism. Cancer : 11: 85-95.
Cardonick, E., Usmami, A. and Ghaffar, S. (2010). Perinatal outcomes of a pregnancy complicated by cancer, including neonatal follow-up after in utero exposure to chemotherapy. Am J Clin Oncol 2010;33:221–8.
Carpenter,Journal of Pediatric. 111(4):507–12.
Centers for Disease Control and Prevention. Lung Cancer: Symptoms. October 22, 2009. Available at http:// www.cdc.gov/cancer/lung/basic_info/symptoms.ht m. Accessed November 12, 2009.
Centers for Disease Control and Prevention. Malignant Mesothelioma Mortality — United States, 1999- 2005. Morbidity and Mortality Weekly Report. April 2009; 58(15):393-6.
Chadha, V. and Shenoi, S.D.(2003). Hair loss in cancer chemotherapeutic patients. Indian J Dermatol Venereol Leprol. 2003; 69: 131-132.
Chen S, Wang, K. andWan, Y.J.(2010). Retinoids activate RXR/CAR-mediated pathway and induce CYP3A. Biochem Pharmacology 79:270–276.
Chen, N., Napoli, J.L.(2008). All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane- associated RARalpha. FASEB J. ; 22:236–245.
Chen, S., Gardner, D.G.(1998). Retinoic acid uses divergent mechanisms to activate or suppress mitogenesis in rat aortic smooth muscle cells. Nature. 15;102(4):653–62.
Cheung-Ong, K., Giaever, G. and Nislow, C. (2013). DNA- damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol. 2013;20(5):648-659.
Cioce, M.and Blandino, G.(2011). PGC1alpha Confers Specificity- Metabolic Stress and p53-Dependent Transcription. Molecular cell ; 44: 515-516.
Clarke, M.F,, Weissmanm,I.L., Wahl, G.M(2006). Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Research.66:9339–9344.
Costanza, M.E. (2001) Epidemiology and risk factors for breast cancer. In: UpToDate. 2001:9:2–3.
Croker, A.K., Allanm, A.L. (2008). Cancer stem cells: implications for the progression and treatment of metastatic disease. Nature 12(2):374–390 del Pino, B.M.(2010).
Chemotherapy-induced Peripheral Neuropathy. NCI Cancer Bulletin. 7: 6. Franklin, D.J. and Packel, L. (2006)
Cancer-related fatigue. Arch Phys Med Rehabil. 87: S91-93. Freter, C.E. and Perry, M.C. (2008). Systemic Therapy. In: Abeloff MD, Armitage JO, Niederhuber JE,
Kastan MB, McKenna WG, eds. Abeloff’s Clinical Oncology. 4th ed. Philadelphia, Pa: Elsevier Churchill Livingstone; 449- 483.
Fuchs-Tarlovsky, V. (2013). Role of antioxidants in cancer therapy. Nutrition. 2013;29(1):15-21.
Greenlee, R.T., Hill-Harmon, M.D., Murry, T. and Thun, M. (2001). Cancer Statistics, 2001. CA Cancer J Clinical. 51: 15
Gullatte, M.M. and Gaddis, J.(2004). Chemotherapy. In: Varrichio CG, ed. A Cancer Source Book for Nurses. 8thed. Sudbury, Mass: Jones and Bartlett; 103-130.
Herceg, Z., Vaissiere, T.(2011). Epigenetic mechanisms and cancer: an interface between the environment and the genome. Epigenetics : 6: 804-819.
Hill, R.P.(2006). Identifying cancer stem cells in solid tumors: case not proven. Cancer Research 66:1891– 1895
Hosoya, N., Miyagawa, K.(2014). Targeting DNA damage response in cancer therapy. Cancer Sci. 105(4):370- 388.
Itano, J.K. and Taoka, K.N. (2005). eds. Core Curriculum for Oncology Nursing. 4th ed. Philadelphia, Pa: Elsevier Saunders;
Jaenisch, R., Bird, A.(2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Genetics; 33 Suppl: 245- 254.
Johnstone, R.W., Ruefli, A.A. and Smyth, M.J. (2000). Multiple physiological functions for multidrug transporter P- glycoprotein? Trends Biochem Sciences 25: 1-6.
Kaestner, S.A. and Sewell, G.J. (2007) Chemotherapy dosing part I: scientific basis for current practice and use of body surface area. Clin Oncol (R Coll Radiol). 19: 23- 37.
Keidan, R.D., Fanning, J., Gatenby, R.A. and Weese, J.L. (2009). Recurrent typhlitis. A disease resulting from aggressive chemotherapy. Disease Colon Rectum. 32: 206-209.
Kirby, J.S. and Miller, C.J. (2010). Intralesional chemotherapy for nonmelanoma skin cancer: A practical review. J Am Acad Dermatol. 2010 May 31. [Epub ahead of print]
Kohn, K. W. (1996). Beyond DNA cross-linking—history and prospects of DNA-targeted cancer treatment— Fifteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 56, 5533–5546.
Kohn, K. W., Spears, C. L., and Doty, P. (1966). Inter-strand crosslinking of DNA by nitrogen mustard. Mol.Biol. 19, 266–288.
Koren, G., Lishner, M. and Santiago, S. (2005). The Motherisk guide to pregnancy and lactation. 2nd ed. Toronto: Motherisk Program; 2005.
Korkaya, H., Paulson, A., Charafe-Jauffret, ,G. and Wicha, M.S. (2009). Regulation of mammary
stem/progenitor cells by PTEN/Akt/β-catenin signaling. Nature 7(6):e1000121.
Kovacic, P. (2007). Unifying mechanism for anticancer agents involving electron transfer and oxidative stress: clinical implications. Med Hypotheses. 69: 510-516.
Lunt, S.Y., Vander, H. (2011). Aerobic glycolysis: meeting the metabolic requirements of cell
Luqmani, Y.A. (2005). Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 14 Suppl 1: 35-48.
Miki, J., Furusato, B., Li, H., Gu, Y.(2007). Identification of putative stem cell markers, CD133 and CXCR4, in hTERTimmortalized primary nonmalignant and malignant tumorderived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Research, 67(7):3153– 3161.
Minchinton, A.I. and Tannock, I.F. (2006). Drug penetration in solid tumours. Nat Rev Cancer. 6: 583- 592.
Missailidis, S. (2008). ed. Anticancer therapeutics. 1st ed. Chichester, GB: John Wiley and Sons, Ltd.; Molofsky, A.V., Pardal, R., Morrison, S.J. (2004). Diverse mechanisms regulate stem cell self-renewal. Nature 16:700–707.
National Academy of Sciences. Biological Effects of Ionizing Radiation (BEIR) VI Report: The Health National Cancer Institute (2015). Chemotherapy Drugs: How They Work. America Cancer society.pp1-17.
Pereg, D., Koren, G. and Lishner, M. (2008). Cancer in pregnancy: gaps, challenges and solutions. Cancer Treat Rev 2008;34:302–12.
Quintana, E., Shackletonm, M., Sabel, M.S. and Fullen, D.R(2008). Efficient tumour formation by single human melanoma cells. Nature 456(7222):593–598.
Reeder, J.G. and Vogel, V.G. (2007). Breast cancer risk management. Clin Breast Cancer 7, 833-40. Rolz-Cruz, G. and Kim, C.C. (2008). Tumor invasion of the skin. Dermatol Clin 26, 89-102.
Rüther, U., Nunnensiek, C. and Schmoll, H.J.(2000). Secondary neoplasias following chemotherapy, radiotherapy, and immunosuppression: Contributions to oncology (Beiträge zur Onkologie) 2000; 55: ISBN 3-8055-7116-X.
Shaikh, A.Y. and Shih, J.A.(2012). Chemotherapy-induced cardiotoxicity. Curr Heart Fail Rep. 9: 117- 127.
Shapira, D. and Urban N. (1991). A minimalist policy for breast cancer Surveillance. JAMA.1991;265:380– 382.
Smith, H., Kammerer-Doakm D., Barbo, D. and Sarto, G.(1996). Hormone Replacement Therapy in the Menopause: A Pro Opinion. CA—A Cancer Journal for Clinicians.46:343.
Smith, I.E. and Dowsett, M. (2003) Aromatase inhibitors in breast cancer. N Engl J Med 348, 2431- 2442.
Soares, D. and Johnson, P. (2007). Breast imaging update. West Indian Med J 56, 351-4. Sonis ST. Oral mucositis in cancer therapy. J Support Oncol.2004; 2: 3-8.
Stead, M.L.(2004). Sexual function after treatment for gynecological malignancy. Curr Opin Oncology, 16: 492-495.
Takahash, N., Breitman, T.R.(1989). Retinoic acid acylation (retinoylation) of a nuclear protein in the human acute myeloid leukemia cell line HL60. Nature 25;264(9):5159–63.
Tatarkova, Z., Kuka, S., Petras, M., Racay, P., Lehotsky, J., Dobrota, D., Kaplan, P.(2012). Why Mitochondria are Excellent Targets for Cancer Therapy. Klin Onkol . 25(6): 421– 426
U.S. Department of Health and Human Services. The Health Consequences of Smoking. A Report of the U.S. Surgeon General. 2004.
Van Calsteren, K., Heyns, L., De Smet, F., Van Eycken, L., Gziri, M.M. and Van Gemert, W. (2010). Cancer during pregnancy: an analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J Clin Oncol 2010;28:683–9.
Vander, H., Cantley, L.C. and Thompson, C.B.(2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science :324: 1029-1033.
Vitiello, D., Naftoilin, F. and Taylor, H.S. (2007) Menopause developing a rational treatment plan.Glycerol Endocrinol 23, 682-91.
Vlastos, G., Kinkel, K. and Pelte, M.F. (2004) Menopause developing a rational treatment plan. Breast Cancer Res Tremd. 88, S16
Yarbro, C.H., Frogge, M.H. and Goodman, M. (2005). Cancer Nursing: Principles and Practice. 6th ed. Sudbury, Mass: Jones and Bartlett;















