1.0 Introduction
Food is an essential ingredient for the good health and sustenance of human life. It’s important, therefore cannot be overemphasized. In most African countries especially Nigeria, people depend mostly on vended food and food from public restaurants for consumption [1]. There is a rise in the patients with foodborne diseases resulting from street vended fruits and vegetables. Food purchased from the food vendors such as restaurants or food hunts is also susceptible to microbial contaminations. Improper washing of fruits and vegetables, untreated human faeces and agricultural bio-solid were major predisposing factors [2].
Adequate education on proper hygiene, decontamination approaches towards improving the quality of fruits and vegetables vended in Nigeria were recommended by most authors. These foods are consumed at the point of sale without any further treatment such as heating [1-2]. In the northern part of Nigeria such as Kaduna foods such as cooked rice, cooked beans, moimoi, fried yam, Salad, eba, Egusi soup, cooked semovita, Beans cake, and Pounded yam are mostly purchased at food vendors. Based on the relatively cheap and easily available of these ready-to-eat foods, their consumption has increased over the few years [3].
Despite the excessive patronage of these food vendors /handlers; it is very essential to ensure the safety level of these foods from contaminants and microorganisms. Over the years Foodborne disease outbreaks linked with foods have been associated with various foodborne pathogens [3, 4]. Foodborne diseases are a major global problem causing considerable morbidity and mortality annually [5].
Increased antimicrobial resistance to most antibiotics prescribed has been reported to be mostly caused by Escherichia coli which are one of the most important opportunistic pathogens ever recorded from humans [6]. Antibiotic resistance in E. coli has become a serious and growing phenomenon in contemporary medicine, and it’s emerging as one of the preeminent public health concerns of the 21st century reported by several studies on bacterial drug resistance especially in E. coli [7]. The magnitude of extraintestinal infections by E. coli was reported to be in the range of 6 to 8 million with 0.1 million cases per year of sepsis in the United States [8], with an increase in antibiotic resistances every- day [9].
Since resistance properties accrue through different routes, such as natural or intrinsic, mutational changes, and acquisition of plasmid or transposons. This study aims to investigate the antibiotic susceptibility of E. coli isolates from some food samples, cooking utensils and palm of food handlers in some restaurants in Zaria metropolis; in order to proffer better treatment option during therapeutics.
2.0 Materials and Method
2.1 Sample Collection
A total of 220 samples of food (cooked rice, cooked beans, moimoi, fried yam, Salad, eba, Egusi soup, cooked semovita, Beans cake, and Pounded yam), seven (7) samples of palms of food handlers was swab including ten (10) plates and thirteen (13) spoons samples were also swab, which was used within the restaurant. All the samples were collected within April to June 2019 from restaurants in five different locations (Samaru, Tudunwada, Sabo Gari, Wusasa, and Kwagila) in Zaria, Kaduna State
2.2 Ethical Clearance
Ethical approval for this work was obtained from the ethical committee of Ahmadu Bello University committee for research in human subject.
2.3 Swab sample collection
The hand-swab, spoon-swab and plate-swab samples were collected by swabbing the palms, spoons, and plates of the food vendors with sterile swab sticks and were transported to the Pharmaceutical Microbiology laboratory for analysis
2.4 Identification of the Isolates
The red colonies were selected from each MacConkey agar plate. Gram staining methods and further biochemical tests were carried out to identify and characterize the organisms that were isolated from the food and cooking utensils samples. Further confirmation was carried out using Microgen test kits [10].
2.5 Antimicrobial Susceptibility testing
The Modified Kirby-Bauer disc diffusion method was used to determine the antibiotic susceptibility of isolates which has been identified and confirmed by biochemical tests. Discrete colonies of isolates on nutrient agar plates were emulsified in 5 ml of sterile physiological saline and the turbidity adjusted to 0.5 McFarland standards (approximately a cell density of 1.5 × 108 CFU/ml). The standardized suspension was inoculated on Mueller Hinton agar using a sterile swab to ensure even distribution and confluent growth. The disc of the various antibiotics was aseptically placed using an antibiotic dispenser, a pre diffusion time of 30 minutes was allowed and the plates were then incubated at 37oC for 18 hours. After incubation, the diameter of the zones of inhibition produced by each antibiotic disc was measured and recorded. The results were interpreted according to the Clinical and Laboratory Standards Institute [11].
The selected antibiotics used for this study are: Ampicillin (AMP, 30µg), Amoxicillin-clavulanic acid (AMC, 30µg), Ceftriaxone (CRO, 30µg), Chloramphenicol (C, 30µg), Ciprofloxacin (CIP, 5µg), Gentamicin (CN, 10µg), Imipenem (IMP, 10µg), Tetracycline (TET, 30µg), and Trimethoprim-sulphamethoxazole (SXT, 25µg) (all from Oxoid Ltd. Basingstoke, London)
2.6 Determination of Multiple Antibiotic Resistance (MAR) Index
The multiple antibiotics resistance (MAR) index was determine for each isolates by dividing the numbers of antibiotics to which the isolates is resistant to by the total number of antibiotics tested Paul et al., [12]
3.0 Results
3.1 Occurrence frequency of E. coli from food samples
The incidence of E. coli isolates from food, cooking utensils and palms of food handler’s samples in Zaria metropolis, Nigeria, after biochemical characterization, using MicrobactTM 12E Gram-negative identification kit. Out of 158 acclaimed Enterobacteriaceae isolates evaluated, 19 % (30) were confirmed to be E. coli, while 81 % were other Enterobacteriaceae organisms such as Klebsiella. spp, Citrobacter fruendii, Enterobacter spp, Shigella spp, Salmonella spp, Serratia spp, Cronobacter sakazaki
3.2 Antibiotic-resistant pattern of E. coli from food samples
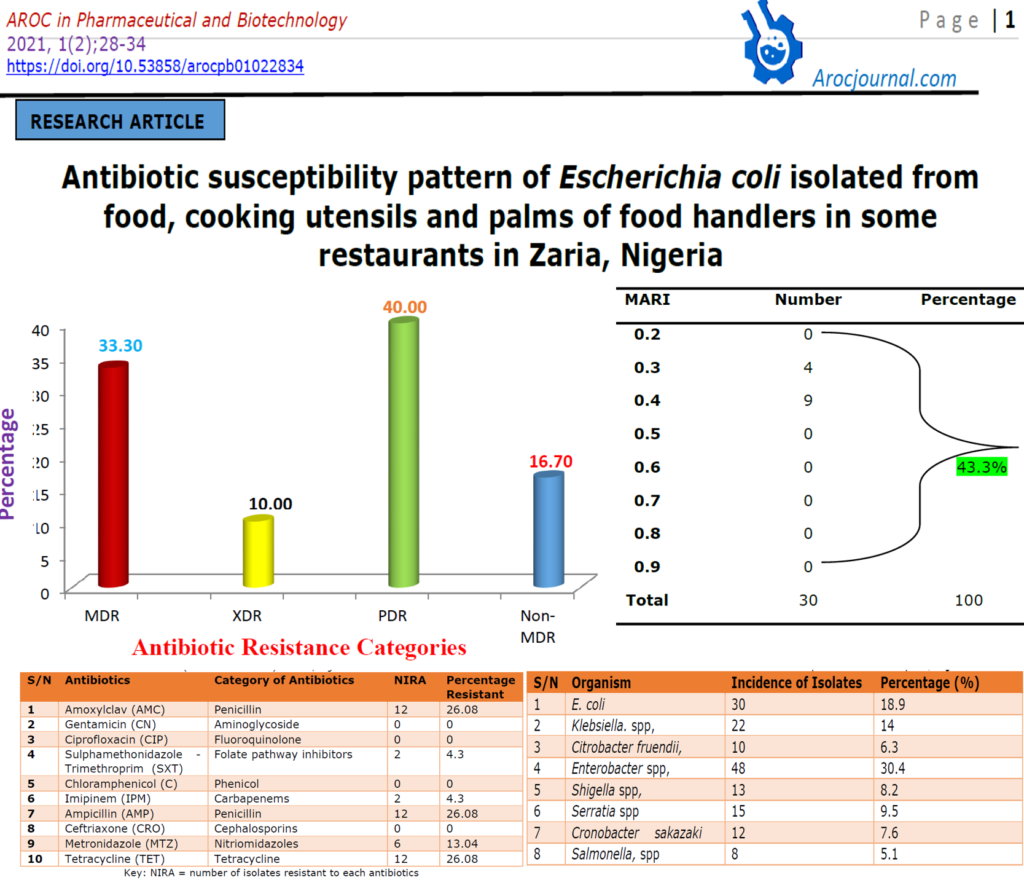
The number of E. coli isolates resistant to each antibiotic and their percentages are shown in table 2 and figure 1. The majority of the isolates were resistant to Amoxylclav (26.08%), Ampicillin (26.08%), and Tetracycline (26.08%) and Metronidazole (13.04%). The isolates were mostly MAR isolates being resistant to at least two of the antibiotics resistance. The multiple antibiotic resistance index of > to 0.2 was shown by 43.3% of E. coli (Table 3)
Table 1: Identification of E. Coli from food samples in Zaria metropolis, Nigeria.
| S/N | Organism | Incidence of Isolates | Percentage (%) |
| 1 | E. coli | 30 | 18.9 |
| 2 | Klebsiella. spp, | 22 | 14 |
| 3 | Citrobacter fruendii, | 10 | 6.3 |
| 4 | Enterobacter spp, | 48 | 30.4 |
| 5 | Shigella spp, | 13 | 8.2 |
| 6 | Serratia spp | 15 | 9.5 |
| 7 | Cronobacter sakazaki | 12 | 7.6 |
| 8 | Salmonella, spp | 8 | 5.1 |
Table 2: Percentage of E. Coli Resistance to Some Common Antibiotics Mostly used for theTreatment of Diarrhea (food borne infection) in Zaria, Nigeria
| S/N | Antibiotics | Category of Antibiotics | NIRA | Percentage Resistant |
| 1 | Amoxylclav (AMC) | Penicillin | 12 | 26.08 |
| 2 | Gentamicin (CN) | Aminoglycoside | 0 | 0 |
| 3 | Ciprofloxacin (CIP) | Fluoroquinolone | 0 | 0 |
| 4 | Sulphamethonidazole – Trimethroprim (SXT) | Folate pathway inhibitors | 2 | 4.3 |
| 5 | Chloramphenicol (C) | Phenicol | 0 | 0 |
| 6 | Imipinem (IPM) | Carbapenems | 2 | 4.3 |
| 7 | Ampicillin (AMP) | Penicillin | 12 | 26.08 |
| 8 | Ceftriaxone (CRO) | Cephalosporins | 0 | 0 |
| 9 | Metronidazole (MTZ) | Nitriomidazoles | 6 | 13.04 |
| 10 | Tetracycline (TET) | Tetracycline | 12 | 26.08 |
Key: NIRA = number of isolates resistant to each antibiotics
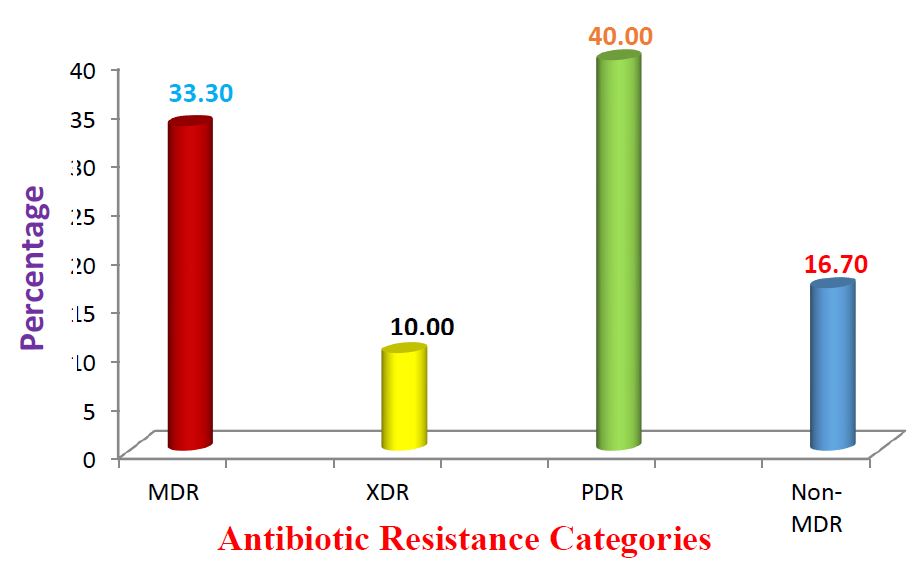
Table 3: Multiple Antibiotic Resistance Index (MARI) of E. coli Isolates from Food samples in Zaria, Nigeria
4.0 Discussion
Foodborne disease is a major public health problem causing considerable morbidity and mortality annually [13]. The isolation of Escherichia coli, Salmonella, spp., Shigella spp., Klebsiella spp., and other Enterobacteriaceae as food contaminants in this study is similar to the studies of Idowu et al., [14] which reported that these microorganisms were implicated in contamination of ready-to-eat foods. Similar findings have also been reported by other researchers [15-17].
Out of 158 acclaimed Enterobacteriaceae isolates evaluated, 19 % (30) were confirmed to be E. coli, while 81 % were other Enterobacteriaceae organisms such as Klebsiella. spp, Citrobacter fruendii, Enterobacter spp, Shigella spp, Salmonella spp, Serratia spp, Cronobacter sakazaki. The majority of the isolates were resistant to amoxiclav (26.08%), ampicillin (26.08%), and tetracycline (26.08%) and metronidazole (13.04%). Thirty-three (33.3%) of the isolates were multidrug-resistant. The E. coli isolates were mostly multiple antibiotic resistance with 43.3% having multiple antibiotic resistance index (MARI) ≥ 0.2
Ciprofloxacin, Chloramphenicol, Ceftriaxone and Gentamicin susceptibility of E. coli isolates (0% overall resistance) in this study proves Ciprofloxacin, Chloramphenicol, Ceftriaxone Gentamicin as one of the possible effective antibiotics. This is corroborated by the findings of Igba et al. [18] in which almost all tested enteric organisms except E. coli identified in his study were 100% sensitive to ciprofloxacin, gentamicin, and trimethoprim-sulphamethoxazole. This means that these antimicrobials are still drugs of choice for the management of foodborne illnesses in this locality of Zaria. On the other hand, E. coli evolved resistance to ampicillin, Amoxylclav, and Tetracycline and other tested antimicrobial drugs which would make the treatment of E. coli infections difficult.
The high percentage (43.4%) of E. coli having MAR index ≥ 0.2 in this study, suggests that the isolates originated from a high-risk source of contamination where antibiotics are often used [19]. However, the percentage of MDR (33.3%) obtained in this study (Figure 1) might be an indication that some proportion of the E. coli isolates have been pre-exposed to several antibiotics. Furthermore, the combination of microbial characteristics such as selective pressure on antimicrobial usage and technological changes that enhance the transmission of drug-resistant organisms might be the cause of this resistance [20]. Other reasons could be due to the increase in transmission of resistant isolates between vendors from food. Therefore, this attribute has made E. coli to be considered an important reservoir of transferable antibiotic resistance [21]. The difficulties in the treatment of food and water associated gastrointestinal diseases due to E. coli have been reported [22]. This problem is compounded by the continued emergence of antibiotic resistance to a growing number of antibiotics [23-24].
5.0 Conclusion
This study showed the presence of microbial contamination of food samples. The presence of multidrug resistance E. coli could serve as an indicator for the need to promote awareness about the possible health hazards that could be due to poor handling of these foods. There is, therefore, the need for agencies to ensure that microbiological standards are established and practised by food sellers for the handling distribution of food.
Conflict of Interest: We have no conflict of interests
Finding: This work receives no external funding
Acknowledgement: The authors wish to acknowledge the staff members of the Department of Pharmaceutical Microbiology, Ahmadu Bello University Zaria.
Author Contributions: Authors V.E. and G.O.A conceived the idea and designed the study, conducted the study, performed data analysis and interpretation, and wrote the first draft of the manuscript. Authors BAT and P.I. contributed data or analysis tools, correction of draft. All authors read and approved the final version to be published.
References
1. Erhirhie, E., Omoirri, M., Chikodiri, S., Ujam, T., Kesiena, E., Oseyomon, J. (2020). Evaluation of Microbial Quality of Vegetables and Fruits in Nigeria: A Review. International Journal of Nutrition Sciences, 5(3), 99-108. doi: 10.30476/ijns.2020.86034.1065
2. Amusa, N. A., & Odunbaku, O. A. (2009). Microbiological and nutritional quality of hawked kunun (a sorghum based non-alcoholic beverage) widely consumed in Nigeria. Pakistan Journal of Nutrition, 8(1), 20-25.
3. Gilbreth SE, Call JE, Wallace FM, Scott VN, Chen Y, Luchansky JB. Relatedness of Listeria monocytogenes isolates recovered from selected ready-to-eat foods and listeriosis patients in the United States. Applied and Environmental Microbiology. 2005;71:8115–8122.
4. Gibbons IS, Adesiyun A, Seepersadsingh N, Rahaman S. Investigation for possible source(s) of contamination of ready-to-eat meat products with Listeria spp. and other pathogens in a meat processing plant in Trinidad. Food Microbiology. 2006;23:359– 366.
5. Hanson LA, Zahn EA, Wild SR, Döpfer D, Scott J, Stein S. Estimating global mortality from potentially foodborne diseases: An analysis using vital registration data. Population Health Metrics. 2012;10:5.
6. Stacy Alyssa K, Natalie M. Mitchell, Jacob T. Maddux, Miguel A. De la Cruz, Laura Durán, Jorge A. Girón, Roy Curtiss 3rd, Melha Mellata Mail (2014). Evaluation of the Prevalence and Production of Escherichia coli Common Pilus among Avian Pathogenic E. coli and Its Role in Virulence. PLoS ONE 9(1): e86565.
7. Islam T.M.d. Sunzid, A. Marufa, N. and Nigarin, S. (2013). Culture and Antibiotic Sensitivity of Escherichia coli Isolated from Patients with Urinary Tract Infections (UTI) in Jessore City. IOSR Journal of Pharmacy and Biological Sciences; 8(5): 66-69
8. Doyle John (2013). Anesthesia for otolaryngologic surgery. Edited by Basem Abdelmalak and D. John: Cambridge University Press. Pp. 282–287.
9. Miranda, S. Davide, M. G. and Peter, J. C. (2004). Evolution of multi-resistance plasmids in Australian clinical isolates of Escherichia coli. Microbiology, 150: 1539-1546
10. Nwankwo, I.U. Eze, V.C. Onwuakor, C.E. Friday, J.U. (2015). Evaluation of the Degree of Contamination of Salad Vegetable Sold in Umuahia Main Market. America Journal of Microbiology Reseach. Vol 3. 41-44
11. Clinical and Laboratory Standard Institute (CLSI) (2016). Performance Standards for Antimicrobial Susceptibility Testing. 26th edition. CLSI document M100-S26. Wayne, PA.
12. Paul, S. B, Roy R. L. M. K., and Ghosh, A.C. (1997). Multiple Antibiotic Resistance (MAR) index and its Reversion in Pseudomonas aeruginosa. Letters in Applied Microbiology. 24: 169- 171.
13. Hanson, L.A. Zahn, E.A. Wild, S.R. Döpfer, D. Scott, J. Stein, S. (2012). Estimating global mortality from potentially foodborne diseases: An analysis using vital registration data. Population Health Metrics. 10:5.
14. Idowu, O.A. (2006). Oral faecal parasites and personal hygiene of food handlers in Abeokuta, Nigeria. Africa Health Science, 6:160–164.
15. Taulo, S., Wetlesen, A. (2008). Abrahamsen R, Mkakosya R, Kululanga G. Microbiological quality of water, associated management practices and risks at source, transport and storage points in a rural community of Lungwena, Malawi. Africa Journal of Microbiological Research. 7(2):131-137.
16. Mepba, H.D. Achinewhu, S.C. Aso, S.N. and Wachukwu, C.K. (2007). “Microbiological quality of selected street foods in Port Harcourt, Nigeria,” Journal of Food Safety, vol. 27(2), pp. 208–218
17. Eni, O.A. Oluwawemitan, I.A. and Solomon, O.U. (2010). “African Microbial Quality of Fruits and Vegetables Sold in Sango Ota, Nigeria. Journal of Food Science, 4(5):291- 296.
18. Igba P. Tytler B. A.1 Hamza, J. A. Adeshina G. O. (2021). Bacteriological Quality of Fresh Vegetables and Peeled Sugar-Cane Obtained from Selected Markets in Zaria, Nigeria. Journal of Natural Sciences Research, 12(8); 222-229
19. Temesgen, E. Haimanot, T. Derese, D. and Gebre, K. (2016).Bacteriological Quality of Street Foods and Antimicrobial Resistance of Isolates in Hawassa, Ethiopia. Ethiop Journal of Health Sci. 26(6): 533–542
20. Christopher, A.J. Hora, S. and Ali, Z. (2013). Investigation of plasmid profile, antibiotic susceptibility pattern multiple antibiotic resistance index calculation of Escherichia coli isolates obtained from different human clinical specimens at tertiary care hospital in Bareilly-India. Annals of Tropical Medicine and Public Health. 6(3): 285-289.
21. Orozova, P., Chikova, V., Kolarova, V., Nenova, R., Konovska, M. and Najdenski, H. (2008). Antibiotic resistance of potentially pathogenic Aeromonas strains. Trakia Journal of Sciences, 6(1): 71-78.
22. Aibinu, I.E., Peters, R.F., Amisu, K.O., Adesida, S.A., Ojo, M.O., and Odugbemi, T., 2007, Multidrug Resistance in E. coli 0157 Strains and the Public Health Implication., Journal of American Sciences, 3(3), 285 – 290.
23. Patoli, A. A. B., Bushra, V. M., Patoli, G., 2010, High pre-valence of multi-drug resistant Escherichia coli in drink ing water Samples from Hyderabad., Journal of Medical Sciences, 8(1), 1- 23.
Goettscha, W, W. vanPelta, Nagelkerkea, N., Hendrixb M. G. R., Buitingc, A. G. M., and Petitd, P. L., 2000, Increas ing resistance to fluoroquinolones in Escherichia coli from uri-nary tract infections in the Netherlands., Journal of Anti mi-crobial Chemotherapy, 46, 222 – 223.