1.0 Introduction
The term ‘maize’ seems to be derived from the word ‘mahiz’ of Taino language of the Caribbean islands, which became ‘maiz’ in Spanish. Based on this common name, Linnaeus included the name as species in the botanical classification of Zea maize is also popularly known as ‘corn’ in English-speaking countries [1].
Maize (Zea Mays L.) belongs to the grain producing monocotyledonous family, the Poacea, the term maize was derived from South American Indian Arawak-Carib word Mahiz [2], The crop was first introduced into Nigeria probably in the 16th century by the Portuguese [3]. Maize (Zea mays L.) is the most important staple food in Africa, and, in parts of West Africa, it may be consumed up to three times daily. Maize is an important cereal in many developed and developing countries of the world, it is widely used for animal feed and industrial raw material such as starches, acids and alcohols in the developed countries whereas the developing countries use it in general for food [4]. Recently, there has been interest in using maize for the production of ethanol in the United States and China, a substitute for petroleum based fuels [5].
In Nigeria maize is the most popular food crop in the domestic market, it fills in for other food crops like sorghum, millet, plantain, yam and cocoyam when they are in short supply; it is the main feedstuff for poultry and other livestock [6].
Preparation and uses of maize alone or in combination with other food material as staple food or snacks in Nigeria include the followings: ogi (in hot and cold forms), tuwo, donkunnu, maasa, Couscous, akple, gwate, nakia, egbo, abari, donkwa, ajepasi, aadun, kokoro, elekute etc [6]. Ivic et al. [7] reported that maize ear rot disease (cob rot) may occur either as a pre-harvest infection or as storage moulds (causing kernel rots) after harvest. Fusarium spp, have been reported to be the two most common causative agents of maize ear rots.
Natural products have served as a source of bioactive agents for the treatment of infectious diseases [8]. Waltheria indica L. belongs to Sterculiaceae family and it is an erect perennial herb. The leaves are stalked with shallow and irregularly toothed margins [9]. The plant has been reported for several pharmacological and biological activities including antioxidants, anti-microbial, anti-parasitic and antidiabetic among others [9, 10]. Senna tora (L.) Roxb. (S. tora) belonging to the family Fabaceae is an annual undershrub which grows all over the tropical countries. It grows well in wasteland as a rainy season weed [11]. Vernonia amygdalina Del., commonly called bitter leaf, is a perennial shrub that belongs to the family Asteraceae and grows throughout tropical Africa [12]. It has a different processing methods [13] and its widely used as an anti-helminth, anti-malarial, laxative, digestive tonic, appetizer, febrifuge and anti-microbial and wounds [14-16].
However, despite the wide range of biological activities of these plants, literature report revealed scanty of information regarding their inhibitory potential against fungal ear rot disease of maize. Therefore, the present field experiment was set up to examine the effect of aqueous extracts of waltheria (W indica), sickle pod (S. tora) and bitter leaf (Vernonia amygdalina) plants in controlling ear rot fungal pathogen of maize
2.0 Materials and Methods
2.1 Study Area
The study was conducted in the year 2019 and 2020 in the months of August and September in Biu Local Government Area Borno State and Girei Local Government Area of Adamawa State. The experiment comprised of two phases which are laboratory and field investigation. The laboratory experiment was carried out at the Laboratory of Plant Science Department Modibbo Adama University (MAU) Yola for the isolation, control and identification of the pathogens in maize, The field experiment was based on the control of the pathogen and evaluation of agronomic performance of two maize varieties which were conducted at two different locations: MAU campus is located on (Latitude 90 22′ 0”N, Longitude 120 33′ 0”E) in Sub-Sudan savanna and Biu Local Government Area (Latitude 100 36′ 39.96”N, Longitude 120 11′ 42.00”E) [17] in northern Guinea savanna ecology of Nigeria in the year 2019 and 2020 respectively.
2.2 Source of Maize Samples
Two hundred (200) seeds each were collected from Damboa (DAM), Chibok (CBK), Askira/Uba (ASU), Hawul (HWL), Biu (BBU), Shani (SHN), Kwaya Kusar (KWY) and Bayo (BAY) Local Government Areas of Borno State after a survey was conducted and identified farmers who produce maize in each local government area and were giving an equal chance using simple random sampling. From each local government area, two locations (town/village) popularly known for production of maize crops were covered, and from each location two farms were identified for the seed collection, the seed samples were collected on the field. The sample size was 160 representing 10% of total population of 3200 maize seeds. Saunders et al. [18] reported that when the population runs into few hundred use 40% or more, when several hundred use 20%, when thousand use 10% and when several thousand use 5% or less.’’ Simple Random Sampling on the other hand, is a method of drawing a sample from the population so that each member of the population is given an equal and independent chance of being selected the seed samples were labeled and put in plastic bags and tightly sealed and stored. The seed samples were taken to Plant Science laboratory for analysis using the adopted method of Baskin et al. [19]
2.3 Sources of Plant Extracts
Fresh roots, stems, leaves and flowers of bitter leaf (Vernonia amygdalina Del), fresh roots, stems, leaves and fruits of sickle pod (Senna tora) and fresh roots, stems, leaves and flowers sleepy morning (Waltheria indica) were collected in Girei Adamawa State as treatment materials for the experiment. Aqueous extracts of each of the plant materials were prepared. Roots, stems leaves and flowers weighed in 20 grams, 40 grams and 60 grams were placed in sterilized beakers and 100ml of distilled water was added to make 20%, 40% and 60% concentrations as described by Yatai et al. [20]
2.4 Plant extracts preparation
Fresh roots, stems leaves, and flowers of bitter leaf test plants were collected, washed with tap water then with sterile distilled water. The roots, stems leaves and flowers were dried under shade and pulverized using mortar and pestle. Aqueous extracts was prepared by separately mixing grounded roots, stems leaves and flowers with distilled water. Roots, stems leaves and flowers weighed in 20 grams, 40 grams and 60 grams were placed in sterilized beakers and 100ml of distilled water was added to make 20%, 40% and 60% extract as described in previous study [21]. The mixtures was then stirred using a sterile glass rod and allowed to stand at room temperature for 24hours. The extracts was further filtered through micro filter for further purification to avoid contamination [21].
2.5 In vitro control study
Petri dishes containing 20ml of potato dextrose agar each were poisoned with Vernonia amygdalina, Casia tora and Waltheria indica roots, stems, leaves and flower extracts at three concentration levels (20 %, 40 % and 60 %). The spores from the preserved culture were dispensed at the centre of the amended dextrose media, the isolate were flooded with 25ml sterile distilled water with the aid of Carmel hair brush , the spores were carefully brushed off and the sporophores decanted into a sterile petri dish, 15ml of sterile water were added and filtered through 0.2×0.2mm nylon mesh to get rid of mycelial fragments, this filtrate containing the spores were adjusted to a concentration of 1×104 spores per ml through the addition of some quantity of sterile distilled water. Petri dishes were sealed with a masking tape and incubated for 72 hours to observe the inhibitory action of the extracts. The experiment was replicated three times for each concentration level and control. Inhibition level was recorded by drawing two lines intersecting at the centre bottom of petri dish. Linear mycelial growth is taking along predetermined lines and the rate of growth determined.
2.6 Experimental Design
For the in vitro control trial, completely randomized design (CRD) with three replications as described previously [21]. The field experiment phase was conducted in Split Plot Design (SPD) with three replications. Treatments consisted of leaf of bitter leaf extract, at three concentration levels (20%, 40% and 60%).
2.7 Statistical Analysis
Data obtained were analyzed statistically using analysis of variance (ANOVA) with Java Memory Profiler (JMP) version 14 Statistical Analysis System (SAS) incorporation Cary NC 1989-2019 statistical programme, means were separated using Student Neuman Keul’s Test (SNK) at (P< 0.001) Observations were presented in tables and pictorial forms using bar charts designed in Microsoft office 2013 excel sheets
3.0 Results and discussion
3.1 Effect of concentration of aqueous extract of V. amygdalina on growth of Fusarium graminearum
Maize seed treated with aqueous extract of V. amygdalina showed very significant (P< 0.005) reduction in growth of Fusarium graminearum. The effect was also presented based the maximum inhibition concentration recorded at the 20 % (20.18), followed by 40 % (20.16) while the least was recorded at 60 % (22.83). However, the analysis showed there is significant difference in inhibition between concentrations of 0 %, 20 %, 40, and 60 % respectively while there is no significant difference in inhibition between concentrations of 40 % and 60 % respectively when compared with the control (Table 1). From the same Table, it also shows the effect of plant part on the pathogen. The analysis revealed that leaf has the highest inhibition (19.59) followed by flower (22.55) and root (29.94). The least inhibition was recorded with stem (28.20). The analysis showed there is significant difference between all the plant parts but however, there is no significant difference between root and stem (Figure I).
Table 1: Interaction of concentration and plant part of V. amygdalina on growth of Fusarium graminearum
| Concentration (%) | Plant Part | |||
| Flower | Leaf | Stem | Root | |
| 0 | 34.18A | 34.21A | 34.21A | 34.21A |
| 20 | 18.31FG | 15.71GH | 24.58 D | 21.71DE |
| 40 | 17.91FG | 13.91H | 24.91CD | 23.91D |
| 60 | 19.80EF | 14.51H | 29.1B | 27.9BC |
| SE± | 1.236 | |||
Means followed by the same letter(s) are not significantly different using Student Neuman Keul’s Test (SNK)
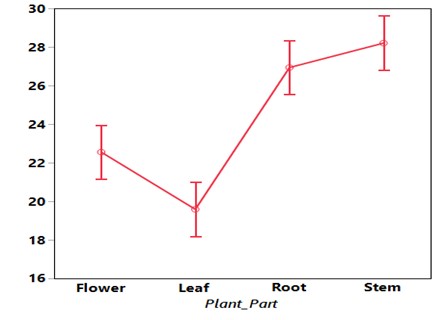
Figure I: Growth of Fusarium graminearum against Plant Parts of V. amygdalina
3.2 Effect of Concentration of Aqueous Extract and Plant Part of W. indica on Growth of Fusarium graminearum
Treatment of seed maize with aqueous extract and plant part of W. indica showed significant (P< 0.005) reduction in growth of Fusarium graminearum. The effect is also shown by the ANOVA analysis of minimum inhibition concentration (Table 2). Maximum inhibition was recorded in 40 % concentration (26.03) followed by 60% concentration (26.10) while the minimum inhibition concentration was recorder at 20 % concentration (28.52). However there is no significant difference in growth between all the concentrations when compared with the control.
On the same table, the analysis of variance showed that leaf has the maximum inhibition (26.19) followed by root (29.02) while flower followed (29.54), the minimum inhibition is recorded in stem (30.77). (Figure II) shows the graphical representation of growth Fusarium graminearumagainst concentration and plants parts of W. indica respectively.
Table 2: Interaction of concentration and plant part of W indica on growth of Fusarium graminearum
| Conc. (%) | Flower | Leaf | Stem | Root |
| 0 | 34.89A | 34.89A | 34.89A | 34.89A |
| 20 | 29.65AB | 27.45BCD | 27.45 BCD | 29.52ABC |
| 40 | 24.05CDE | 20.72E | 30.99AB | 28.39BCD |
| 60 | 29.59AB | 21.72E | 29.79AB | 23.32DE |
Means followed by the same letter(s) are not significantly different using Student Neuman Keul’s Test (SNK)
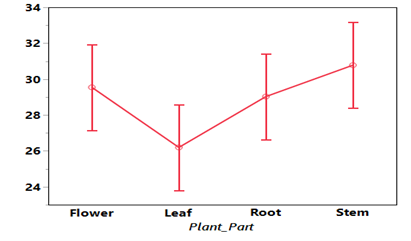
Figure. II: Growth of Fusarium graminearum against Plant Parts of W indica
3.3 Effects of Aqueous Extract and Plant Part of S. tora on Growth of Fusarium graminearum
Seed maize treated with concentration of S. tora extracts does not show significant reduction in the of Fusarium graminearum (P≥ 0.005). Table 10 showed that the maximum inhibition was observed at the concentration of 20% (23.71) followed by 60% concentration (26.44), the minimum inhibition was recorded on 40% concentration (27.74). The result of the analysis showed that there is significant difference 20% (23.71), 40% (27.74) and 60% (26.44). Similarly there is no significant difference between 40% (27.74) and 60% (26.44) respectively when compared with the control. In the same vein the result of the plant parts showed no significant difference. (Figure III) showed the graphical representation of growth of Fusarium graminearum against concentration and plant parts of S. tora respectively.
Table 3: Interaction of concentration and plant part of S. tora on growth of Fusarium graminearum
| Concentration (%) | Plant Part | |||
| Seed | Leaf | Stem | Root | |
| 0% | 32.65 | 32.65 | 32.65 | 32.65 |
| 20% | 22.83 | 25.15 | 21.83 | 20.03 |
| 40% | 32.89 | 28.16 | 24.89 | 25.03 |
| 60% | 29.49 | 25.56 | 24.49 | 26.23 |
| SE± | 2.002 | |||
Means followed by the same letter(s) are not significantly different using Student Neuman Keul’s Test (SNK)
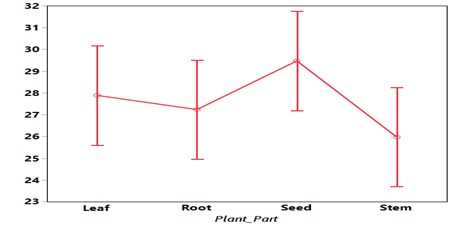
Figure. 3: Growth of Fusarium graminearum against plant parts of C. tora
4.0 Conclusion
It was concluded from the study that Fusarium graminearum is the common pathogenic fungi which cause ear rot disease of maize in the study area. The inhibitory effect of the plant extract against fungal isolate could be due to the presence of antifungal substances present in the extract. Higher inhibition of fungal growth was observed at high concentrations of the aqueous extract. The results of the present investigation are clear indications of the potential of plant extracts to control fungal pathogens. The results also showed that plant extracts significantly reduced the radial growth of isolated fungi and this finding may be the first of its kind in southern part of Borno state
Authors Contributions: The work was conducted in collaboration of all authors. All authors read and approved the final version of the manuscript
Conflicts of interest: The authors declare that they have no competing interests.
Acknowledgement: Nil.
References
1. Ado, S., I. Usman, and U. Abdullahi. Recent development in maize research at Institute for Agricultural Research, Samaru, Nigeria. in 8th African Crop Science Society Conference, El-Minia, Egypt, 27-31 October 2007. 2007. African Crop Science Society.
2. Olaniyan, A.B., Maize: Panacea for hunger in Nigeria. African Journal of Plant Science, 2015. 9(3): p. 155-174.
3. ABIMBOLA, B., Effects of different weed control methods on weeds, growth and yield of maize (zea mays l.) under rainforest ecology, south-west, nigeria. fuoye journal of agriculture and human ecologY, 2019. 1(2).
4. Abdulsalaam, S. and K. Shenge, Seed borne pathogens on farmer-saved sorghum (Sorghum bicolor L.) seeds. Journal of stored products and postharvest research, 2011. 2(2): p. 24-28.
5. Murdia, L., et al., Maize utilization in India: An overview. American Journal of Food and Nutrition, 2016. 4(6): p. 169-176.
6. Asante, D.-B., et al., Antidiabetic effect of young and old ethanolic leaf extracts of Vernonia amygdalina: A comparative study. Journal of diabetes research, 2016. 2016.
7. Abdulrahaman, A. and O.M. Kolawole, Traditional preparations and uses of maize in Nigeria. Ethnobotanical Leaflets, 2006. 2006(1): p. 23.
8. Gwinn, K.D., Bioactive natural products in plant disease control. Studies in natural products chemistry, 2018. 56: p. 229-246.
9. Kannan, M., T.S. Kumar, and M. Rao, Antidiabetic and antioxidant properties of Waltheria indica L., an ethnomedicinal plant. International Journal of Pharma Research and Health Sciences, 2016. 4(5): p. 1376-1384.
10. Madaki, F., et al., Antioxidant potential of Waltheria Indica methanol leaf extract in Trypanosoma Brucei Brucei infected mice. 2019.
11. Pawar, H.A. and K.G. Lalitha, Extraction, Characterization, and Molecular Weight Determination of<i> Senna tora</i> (L.) Seed Polysaccharide. International Journal of Biomaterials, 2015. 2015: p. 928679.
12. Ifeoma, I.I. and E.E. Chukwunonso, Current perspectives on the medicinal potentials of Vernonia amygdalina Del. Journal of medicinal plants research, 2011. 5(7): p. 1051-1061.
13. Tsado, A., et al., Effect of different processing methods on nutritional composition of Bitter Leaf (Vernonia amygdalina). IOSR Journal of Pharmacy, 2015. 5(814.44).
14. Lawal, B., et al., Potential antimalarials from African natural products: A reviw. Journal of intercultural ethnopharmacology, 2015. 4(4): p. 318.
15. Igile, G.O., et al., Flavonoids from Vernonia amygdalina and their antioxidant activities. Journal of Agricultural and Food Chemistry, 1994. 42(11): p. 2445-2448.
16. Alara, O.R., et al., Phytochemical and pharmacological properties of Vernonia amygdalina: a review. Journal of Chemical Engineering and Industrial Biotechnology, 2017. 2(1): p. 80-96.
17. Mayomi, I., J. Abdullahi, and A. Dami, Terrain Analysis of Biu Plateau, for Road Transport Development, Borno State, Nigeria. Journal of Geography and Geology, 2014. 6(2): p. 28.
18. Saunders, M., Research Methods for Business Students (6th edn. 2014.
19. Baskin, C.C. and J.M. Baskin, Seeds: ecology, biogeography, and, evolution of dormancy and germination. 1998: Elsevier.
20. Yatai, A.N., C.M. Ngozi, and E.B. Chukwujindu, Field evaluation of some varieties/accessions of maize for their performances in a derived Savanna Belt of Nigeria. World Journal of Agricultural Research, 2020. 8(4): p. 105-113.
21. Duncan, K.E. and R.J. Howard, Biology of maize kernel infection by Fusarium verticillioides. Molecular plant-microbe interactions, 2010. 23(1): p. 6-16.












