1.0 Introduction
The Jute mallow (Corchorus olitorius) plant belongs to the family of Tiliaceae, the order of Malvales. It is an erect dicot plant found in the wild, but also largely cultivated in Africa and Asia as a green leafy vegetable [1]. In Nigeria, the leaves of jute mallow are mostly consumed as soup across the geopolitical zones. The cooked leaves form a slimy sticky soup like okra that aids easy consumption of starchy foods such as “amala”, “eba”, “semo” and “tuwo”. Jute mallow is called “ewedu or oyoyo”, “ahingbara” and “kama” among the Yoruba, Igbo and the Hausa tribes of Nigeria respectively.
Jute mallow (Corchorus olitorius) is rich in enormous amounts of water and an appreciable number of mineral elements like iron, calcium, potassium, sodium and phosphorous. It contains ascorbic acid, tocopherol, thiamine, riboflavin, niacin and little amount of protein, fat, carbohydrate, fibre, and ash [1-3]. These nutritional constituents justified its usage traditionally in the management of inflammatory conditions, fever, enteritis, dysentery, hypertension and pile [1,4-6]. The nutritional constituents of jute mallow vary greatly based on methods of growing and processing [7-9]. In Nigeria, the fresh jute mallow leaves are usually tenderized for easy pulverization into a semi-consistent puree using a traditional utensil (broom), called “ijabe” in Yoruba land. This tenderization has always been done using potash (potassium bicarbonate). The use of baking soda (sodium bicarbonate) as a substitute is the new trend among millennials.
Potash (potassium bicarbonate) is a dry and hydrated lake salt that exists as white or a mixture of grey/reddish brown with a wide range of particle size [10,11]. It is of low potassium concentration compared to sodium [12,13]. Potash is a common additive in Asian, Oceanian, South American and Sub-Saharan African cuisines [11]. Potash, known as “Kaun” (Yoruba), “Akanwa” (Igbo) or “Kanwa” (Hausa) is the second most commonly used salt in Nigeria [14,15].
Potash helps to reduce cooking time, serves as a tenderizing agent in leguminous and vegetable dishes preparation. It helps to improve the green colouration and remove the bitterness of some vegetables. It also helps to ease the removal of cereal peels, improve the taste and the texture of some foods [11, 16-18]. Sometimes, potash is present as a constituent of herbal concoctions used in cough treatment, relief of constipation, stomach, and toothache [10,19].
However, the adverse effect of potash on the liver, kidney and heart is of great concern [12,19,20]. Excessive and prolonged consumption of potash can cause severe and irreparable damage to these vital organs of the body thereby disrupting normal body functions [15,20,21]. A high concentration of potash suppresses ovarian steroidogenesis, induce abortions due to its ability to increase uterine contractility in the early stages of pregnancy [15,22]. Potash has also been implicated in the incidence of peripartum cardiac failure (PPCF), a condition that occurs during the last months of pregnancy or within few months after delivery [11,15]. Thus, the focus of this study is to find an alternative to these chemical additives whose main function is to soften and reduce the cooking time of jute mallow leaves, thereby making the crushing of the leaves into a fine, smooth homogenized slimy puree easy during preparation.
2.0 Materials and methods
2.1 Samples collection
Jute mallow leaves (Figure 1), potash and baking soda were bought from Gwagwalada market in the Federal Capital Territory (F.C.T), Abuja, Nigeria.
2.2 Sample preparation and cooking
The leaves were prepared based on modified methods [23-25]. The leaves were detached from the stalks, then thoroughly washed under tap water before using distilled water and wrung out. The washed leaves were chopped into tiny bits then divided into four groups of 100 g each (Table 1). Group A is the chopped jute mallow leaves boiled in 300 ml of water for 15minutes. Group B contain the chopped jute mallow leaves boiled in 300 ml of water to which 1 g of potash was dissolved at 80 oC before the addition of the leaves. Group C has the same constituents as B except that baking soda was substituted for potash. The 100 g of jute mallow leave in group D were milled in 200 ml of distilled water with an electric machine (Silver Crest multifunction blender robots, SC-1589) for 3 minutes. The resulting slurry was poured into 100 ml of 80 oC water and cooked for 15 mins. Following cooking, Group A-C were pulverized with the aid of “ijabe”, the traditional utensil (broom).
Table 1. Experimental design
| Samples | Raw materials and processing | ||||
| Jute mallow leaves (g) | cooking water (ml) | Additives(g) | Cooking time (min) | Means of pulverization | |
| Group A | 100 | 300 | – | 15 | “ijabe” |
| Group B | 100 | 300 | Potash | 15 | “ijabe” |
| Group C | 100 | 300 | Baking soda | 15 | “ijabe” |
| Group D | 100 | 300 | – | 15 | blender |
Group A = Jute mallow leaves; Group B = Jute mallow leaves + 1 g of potash; Group C = Jute mallow leaves + 1 g of baking soda; Group D = Jute mallow leaves
2.3 Analysis of proximate compositions
The moisture content, crude protein, crude lipid, crude fibre, ash and carbohydrate (by difference) were determined according to the standard methods of the Association of Official Analytical Chemists (AOAC) [26]. The moisture content of the soup samples was determined by drying 2 g of sample to a constant weight at 105 °C for 3hours in a vacuum oven. Percentage moisture content was calculated as the difference in weight before and after drying.
Crude protein was determined by transferring 2 g of sample into a micro-Kjeldahl flask, 20 ml of conc. H2SO4 and 0.1g of SeO2 were added. The flask was heated on the digestion block at 100 °C under a fume hood until a clear greenish solution appeared. After cooling, 10 ml portion of the digested mixture was distilled with 20 ml of 40% NaOH. The distillate was collected into 20 ml of boric acid and titrated to a pink endpoint with 0.01 M of H2SO4 to obtain the nitrogen content of the distillate. The nitrogen content value was multiplied by a conversion factor (6.25) to obtain crude protein content. Crude lipid was determined with 2 g of each of the soup samples and 120 ml of petroleum ether in the reaction that ran for 5 hours.
The crude fibre was determined with 1 g of defatted soup sample digested with 150 ml of pre-heated H2SO4 and 150 ml of pre-heated NaOH. The washed digested sample was dried at 100 °C overnight, cooled in a desiccator and weighed (W1) before ashing at 550 °C for 2 hours in a muffle. Ashed samples were cooled in a desiccator, weighed (W2) and percentage crude fibre calculated as (Digested sample (W2) – Ashed sample/ weight of sample) 100. Ash content was determined using 2 g of each of the soup samples. The weighed samples were ashed for 3 h at 600 °C in a muffle furnace, cooled in the desiccator and weighed. Percentage ash was calculated by (weight of the ashed sample(W2)/ initial weight of the sample (W1)) 100.
Percentage Carbohydrate content was evaluated as difference between 100 and summation of other proximate parameters; carbohydrates (%) = 100- (CF + CP + CL + A + M). where; CF = Crude Fibre, CP= Crude Protein, M = Moisture, CL= Crude Lipid and A = Ash.
2.4 Analysis of minerals compositions
Magnesium, sodium, calcium and potassium contents were determined using Thermo Scientific Atomic Absorption Spectrometry (AAS) on an AAS ICE 3000 Thermo Scientific spectrometer as described by Perkin-Elmer [27]. The samples were ashed at 500 °C, digested in 5 ml of 20% HCl and the mineral concentrations were determined according to different standard conditions of the elements. Concentrations were extrapolated from standard curves prepared for each of the elements and expressed as mg/100 g.
2.5 Viscosity determination
Viscosity was determined according to the manufacturer’s instruction with NDJ-5S Viscometer. Using 120 ml of the sample, viscosity was measured at ambient temperature with Rotor number 2# at a speed of 6 revolutions per minute.
2.6 Analysis of Vitamin C compositions
The vitamin C content was determined by the iodine titration method according to the modified methods of Ekpa, [28] and Helmenstine, [29]. From each group, 20 g of the soup was frozen and thawed to separate the liquid portion of the soup for the ease of straining out using a Muslin cloth. The liquid extract collected from the soup (5 ml) was made up to 25 ml with distilled water. Iodine Solution (0.01 g/ml) was prepared by dissolving 5 g of potassium iodide (KI) and 0.268 g of potassium iodate (KIO3) in 200 ml of distilled water, 30 ml of H2SO4 was added. The solution was thoroughly mixed and made up to 500 ml with distilled water. Vitamin C (1 mg/ml) was used as a standard solution. The iodine solution was titrated against 25 ml of the vitamin C standard to which ten drops of 1 % starch solution was added and 25 ml of the soup extracts respectively until the onset of a blue-black colour that persisted for 20 seconds. The titre values were recorded and the vitamin C content of the samples was calculated using the equation;
Titre volume of standard vitamin C/ weight of vitamin C = Titre volume of the sample/volume of the vitamin C in the sample.
2.7 Statistical analysis
Analysis was carried out in three replicates for all determinations. The results of three replicates were expressed as mean ± SD. Data were exported from excel and analyzed by Graph pad prism version 8. Analyses of variance, followed by Tukey’s multiple comparisons test were used for the responses. Values were considered statistically significant at p < 0.05 (confidence level = 95%).
3.0 Results and Discussion
3.1 Proximate Compositions
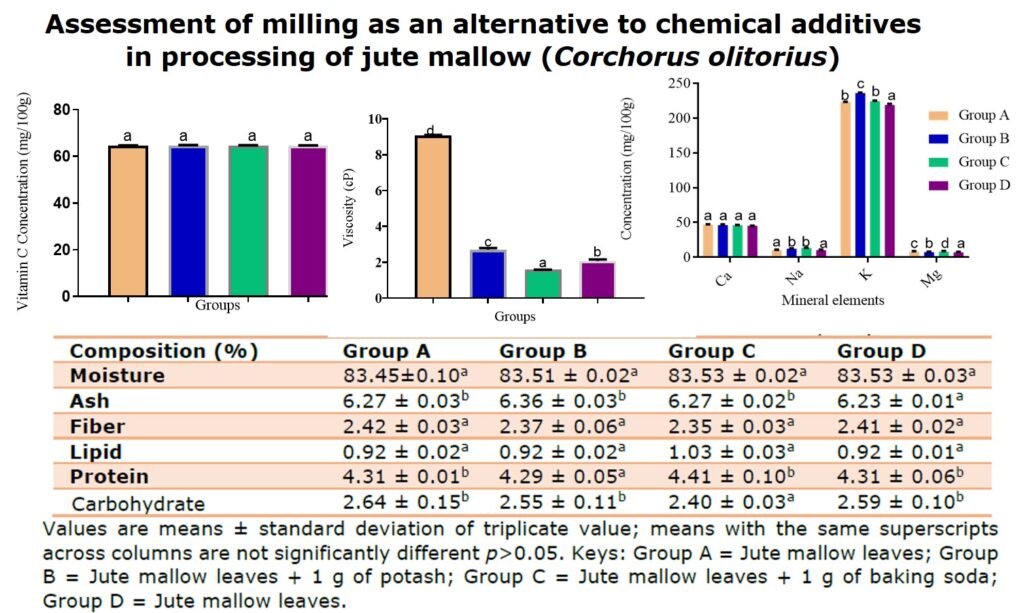
The proximate composition of jute mallow soup under different processing methods is shown in Table 2. There was no significant difference (p>0.05) in the proximate contents of the four groups for moisture, Lipid and fibre. However, groups B, C and D showed a significantly lower (p>0.05) value of protein content (4.29 ± 0.05 %), carbohydrate content (2.40 ± 0.03 %) and Ash content (6.23 ± 0.01 %) respectively. About 84% of moisture content was recorded compared to other nutrients. Although, it was lower than the 92% value reported for cooked jute mallow [24,30]. The variation in the proximate analysis composition could be due to the growing condition, post-harvest condition, method of processing, varieties and the maturation of jute mallow [9,31].
Table 2. Proximate composition of jute mallow (Corchorus olitorius) soups.
| Composition (%) | Group A | Group B | Group C | Group D |
| Moisture | 83.45±0.10a | 83.51 ± 0.02a | 83.53 ± 0.02a | 83.53 ± 0.03a |
| Ash | 6.27 ± 0.03b | 6.36 ± 0.03b | 6.27 ± 0.02b | 6.23 ± 0.01a |
| Fiber | 2.42 ± 0.03a | 2.37 ± 0.06a | 2.35 ± 0.03a | 2.41 ± 0.02a |
| Lipid | 0.92 ± 0.02a | 0.92 ± 0.02a | 1.03 ± 0.03a | 0.92 ± 0.01a |
| Protein | 4.31 ± 0.01b | 4.29 ± 0.05a | 4.41 ± 0.10b | 4.31 ± 0.06b |
| Carbohydrate | 2.64 ± 0.15b | 2.55 ± 0.11b | 2.40 ± 0.03a | 2.59 ± 0.10b |
Values are means ± standard deviation of triplicate value; means with the same superscripts across columns are not significantly different p>0.05. Keys: Group A = Jute mallow leaves; Group B = Jute mallow leaves + 1 g of potash; Group C = Jute mallow leaves + 1 g of baking soda; Group D = Jute mallow leaves.
3.2 Minerals compositions
Figure 1 shows the calcium (Ca), sodium (Na), potassium (K) and magnesium (Mg) composition of the different jute mallow (Corchorus olitorius) soups. The potassium content in these soups was relatively high, approximately 5 folds greater than calcium in this order, K>Ca>Na>Mg. There was no significant difference (p>0.05) in the calcium and magnesium contents across the four groups of soup. Group C (13.54 mg/100g) and B (12.15 mg/100g) showed a significantly higher (p<0.05) sodium content than A (10.44 mg/100g) and D (10.37 mg/100g). This may be a result of the chemical additives (potash in B and baking soda in C) since potash contains more sodium than potassium [19]. The potassium content varies significantly (p<0.05) across the groups in the following group order, B>A=C>D with corresponding values of (235.88, 223.28, 224.17,2 18.95) mg/100g respectively. The higher potassium content observed in group B compared to group A may be as a result of the potash added as a tenderizer.
Increasing dietary intake of potassium reduces blood pressure, decrease cardiovascular disease risk, contribute to bone mineral density, and attenuate the negative consequences associated with consuming high amounts of sodium [32]. Jute mallow is a good dietary source of potassium. However, potassium balance is of enormous importance in maintaining the sodium-potassium pump to prevent hypokalemia or hyperkalemia. Excessive dietary intake of potassium rarely causes hyperkalemia, as it is rapidly excreted by the kidneys [33]. However, in patients with renal disorders, an increase in dietary potassium compounded with the addition of potash may result in hyperkalemia.
The mineral content recorded in this work follows the trend reported by Samuel et al. [30]. Cooking methods can either improve or reduce the nutritional quality of foods [34]. Thus, a relatively lower constituent of mineral elements observed in group D may be accounted for by the larger surface area of the pulverized leaves exposed to cooking heat unlike in the other groups that were cooked before pulverization.

3.3 Viscosity of jute mallow
Figure 2 presents the resistance to flow observed among the four different groups of jute mallow leaf soups. Viscosity observed across the groups was highly significant (p<0.05) in this order, A>B>D>C. Group A recorded the highest viscosity o approximately 9 cP compared to (2.7, 1.6 and 2.1) cP in groups B, C and D respectively. The difference in viscosity observed across the groups was due to variation in their degrees of tenderization. Heat alone could not soften the jute mallow leaf in Group A within the period of cooking, this hindered its pulverization and contributed to its viscosity. Group C was the least viscose and draws better than all, meaning baking soda was able to soften the leaves better than the age-long potash. Group D that was pulverized before cooking was however more viscose and draws lesser than group C, perhaps baking soda does not only soften but enhances the drawing ability of the jute mallow leaves soup
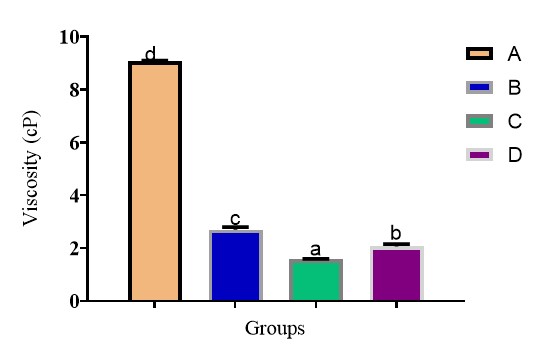
Figure 2. Viscosity of jute mallow (Corchorus olitorius) soups. Bars with different superscript letter are significantly different p<0.05. Group A = Jute mallow leaves; Group B = Jute mallow leaves + 1 g of potash; Group C = Jute mallow leaves + 1 g of baking soda; Group D = Jute mallow leaves
3.4 Ascorbic acid compositions of jute mallow
The ascorbic acid content of jute mallow among the four groups of soups (Fig. 3) shows that there was no observed significant difference (p>0.05) in the vitamin C content of the soups across the four groups, meaning neither of the processing methods impacted the vitamin C contents. The vitamin C content in this work is within the recommended dietary allowance (RDA) of 45 to 90 mg daily [35] and the range reported by Islam [1], Ogunlesi [36]. Vitamin C deficiency causes scurvy, a disorder whose symptoms include bleeding gums, swollen and painful legs, bruising, skin haemorrhages, weakness, and apathy [35]. It is found in varying quantities in fruits and vegetables. It scavenging activity help to prevent tissue damage, aid speedy recovery of cold, cough, influenza, sores, wounds, gingivitis, skin diseases, diarrhoea, malaria and bacterial infections [36].
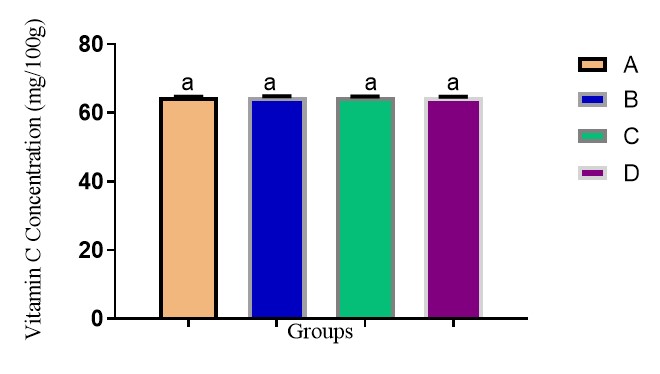
Figure 3. Vitamin C content of jute mallow (Corchorus olitorius) soups. Bars with the same letter are not significantly different p>0.05. Group A = Jute mallow leaves; Group B = Jute mallow leaves + 1 g of potash; Group C = Jute mallow leaves + 1 g of baking soda; Group D = Jute mallow leaves
4.0 Conclusion.
From the parameters examined in this work, it can be concluded that milling before cooking can be adopted as a means of cooking jute mallow leaves soup without the addition of chemical additives which can pose serious health risks following accumulation.
Funding: This work received no external funding.
Competing Interests: The authors declared that no conflict of interest exists.
Ethical Approval: Not applicable
Authors contributions: All authors were involved in intellectual content, literature search, manuscript preparation, editing and review. All authors read and approved the final submission of the manuscript
References
- Islam, M. M. (2013). Biochemistry, Medicinal and Food values of Jute (Corchorus capsularis L. and C. olitorius L.) leaf: A Review. International Journal of Enhanced Research in Science Technology & Engineering, 2(11), 35–44.
- Nyadanu, D., Amoah, A. R., Kwarteng, A. O., Akromah, R., Aboagye, L. M., Adu-Dapaah, H., Dansi, A., Lotsu, F., and Tsama, A. (2017). Domestication of Jute Mallow (Corchorus olitorius L.): Ethnobotany, Production Constraints and Phenomics of Local Cultivars in Ghana. Genetic Resources and Crop Evolution, 64(6), 1313-1329. DOI 10.1007/s10722-016-0438-4
- Agomuo, J. K., Okache, T. A., and Abdulrrauf, A. (2021). Effect of Drying Methods on the Physicochemical and Sensory Properties of Jute (Corchorus Olithorius) Leaves. FUDMA Journal of Agriculture and Agricultural Technology, 6(2), 160–166.
- Ali, M., Ray, B., Rokeya, B., Aleya, M., and Ahmed, Z. (2019). Potential Healing Powers with Jute Plant- A Review. International Journal of Sciences: Basic and Applied Research (IJSBAR), 48(5), 10–23.
- Isuosuo, C. C., Akaneme, F. I., and Abu, N. E. (2019). Nutritional Evaluation of the Seeds of Corchorus olitorius: A Neglected and Underutilized Species in Nigeria. Pakistan Journal of Nutrition, 18(7), 692–703. https://doi.org/10.3923/pjn.2019.692.703
- Obode, O. C., Adebayo, A. H., Omonhinmin, C. A., and Yakubu, O. F. (2020). A Systematic Review 0f Medicinal Plants Used in Nigeria for Hypertension Management. International Journal of Pharmaceutical Research, 12(4), 2231–2276. https://doi.org/10.31838/ijpr/2020.12.04.142
- Adediran, O. A., Ibrahim, H., Tolorunse, K. D., and Gana, U. I. (2015). Growth, Yield and Quality of Jute Mallow (Corchorus olitorius L.) as Affected by Different Nutrient Sources. International Journal of Agriculture Innovations and Research, 3(5), 2319–1473.
- Ayodele, O. P., Aluko, O. A., and Adegbaju, O. D. (2021). Effects of Catnip (Nepeta cataria L.) and Mexican Sunflower (Tithonia diversifolia L.) Density on Growth, Yield, and Proximate Composition of Jute Mallow (Corchorus olitorius L.). Plant Varieties Studying and Protection, 17(2), 155–163
- Olubanjo, O. O., Adaramola, O. D., and Alade, A. E. (2021). Development of Greenhouse with Root Dipping Technique Hydroponics Structure to Test the Performance of Jute Mallow. European Journal of Agriculture and Food Sciences, 3(4), 10–18.
- Momoh, T. B., Yaro, C. A., Usman, S. O., and Iyeh, V. A. (2019). Effect of Potash on the Tenderness and Phytochemical Constituents of Cajanus cajan. Trends in Applied Sciences Research, 14(4), 278–282. https://doi.org/10.3923/tasr.2019.278.282
- Kégah Ngoualem, F., Nguimbou, R. M., Muhammad Khan, A., and Ndjouenkeu, R. (2020). Chemistry and Functionalities of Lake Deposits and Plant-Based Salts Used in Food Preparations: A review. Food Chemistry, 321(November 2018), 126674. https://doi.org/10.1016/j.foodchem.2020.126674
- Kutshik, R. J., Idogun, F. O., Ujah, F. E., and Gotom, S. S. (2018). Comparative Investigations of the effects of Kanwa (Trona) and Tokansenyi (Plant Potash) on Liver and Kidney of Albino Rats. International Journal of Biological and Chemical Sciences, 12(1), 422-430. https://doi.org/10.4314/ijbcs.v12i1.33
- Nata’ala, M. K., Mormoni, A. B., Isah, A. A., Ibrahim, U. B., Zauro, S. A., and Halilu, M. E. (2018). Potency of Trona on Fungi Associated with Tinea Capitis from Children in Usmanu Danfodiyo University Sokoto (UDUS). South Asian Journal of Research in Microbiology, 2(1), 1–6. https://doi.org/10.9734/sajrm/2018/v2i129241
- Alaribe, C. S., Oladipupo, A. R,. Gbadamosi, S., Emuejevoke, T. T., and Bashiru, K. A. (2019). Determination of Metals by Atomic Absorption Spectrometry in Unripe Plantain Peels Ash and Comparative Genotoxicity Studies with Kaun “Potash” Using Gel Electrophoresis and Diphenylamine Assay. J. Chem Soc. Nigeria, 44(3), 418–425.
- Ojo, C. A. (2021). Effect Of Ash Produced from Different Agricultural Residue as an Alternative for Natron on Human Health. Journal of Agricultural Science, 10(2), 43-47.
- Uzogara, S. G., Morton, I. D., and Daniel, J. W. (1992). Processing, Microstructural and Nutritional Changes in Cowpeas (Vigna Unguiculata) Cooked in Kanwa Alkaline Salt. In: Proceedings of a Regional Workshop on Traditional African Foods Quality and Nutrition, Dar es Salaam, 3-5 November 1992, 18.
- Ekosse, G. I. E. (2010). X-ray diffraction study of kanwa used as active ingredient in achu soup in Cameroon. African Journal of Biotechnology, 9(46), 7928–7929. https://doi.org/10.5897/ajb09.1805
- Ajiboye, T. O., Komolafe, Y. O., Yakubu, M. T., and Ogunbode, S. M. (2015). Effects of Trona on the Redox Status of Cellular System of Male Rats. Toxicology and Industrial Health, 31(2), 179–187. https://doi.org/10.1177/0748233712469654.
- Imafidon, K. E., Egberanmwen, I. D., and Omoregie, I. P. (2016). Toxicological and Biochemical Investigations in Rats Administered “Kaun” (Trona) A Natural Food Additive used in Nigeria. Journal of Nutrition and Intermediary Metabolism, 6, 22–25. https://doi.org/10.1016/j.jnim.2016.05.003
- O. K., Ndukaku, O. Y., Ifeanyi, O. E., and Ndubuisi, O. T. (2014). Effect of Potash on the Hearts of Rabbits. IOSR Journal of Pharmacy and Biological Sciences, 9(5), 33–38. https://doi.org/10.9790/3008-09523338
- Bankole J. K., Ngokere A. A., Ajibade O. M., Igunbor C. M. and Eloka C. C. V. (2015). Degenerating effects of potash (Kaun-K2CO3) on the kidney: Unabated Continental Challenge to Human Health in Nigeria. Scholars Research Library Annals of Biological Research, 6(3), 12–18. http://scholarsresearchlibrary.com/archive.html
- Rabiu, A. and Malami, S. (2019). Toxicity Study of Potash Extract, “jar Kanwa”: An Earthy Material Consumed for Remedy of various Ailments in Northern Nigeria. Journal of Biomedical Research and Clinical Practice, 2(4), 197–201.
- Musa, A., and Ogbadoyi, E. O. (2012). Effect of Cooking and Sun Drying On Micronutrients, Antinutrients and Toxic Substances in Corchorus Olitorius (Jute Mallow). Journal of Nutrition & Food Sciences, 02(03), 1-8.
- Abdalla, M. M., Attia, M., Yousef, M. I. and Abd el-Aal, M. H. (2016). Effect of Cooking on Nutritive Value of Jew’s Mallow (Corchorus olitorius L.) and Mallow (Malva parviflora L.) Leaves. Alexandria Journal of Food Science and Technology, 13(2), 1–10. https://doi.org/10.12816/0038408
- Traoré, K., Parkouda, C., Guissou, A. W. D. B., Traoré, Y., and Savadogo, A. (2019). Effect of Cooking Methods on the β -carotene Content of Jute Mallow Leaves (Corchorus olitorius). American Journal of Food Science and Technology, 7 (6),223-226. DOI: 10.12691/ajfst-7-6-9
- AOAC (2010) Official Methods of Analysis of Association of Official Analytical Chemists. 18th Edition, Washington, DC.
- Perkin-Elmer (1996) Analytical Methods for Atomic Absorption Spectrophotometry. Perkin-Elmer Corporation, USA.
- Ekpa, E., Folake O.M., and Adeoye R. (2016). Effect of Processing Methods and Time on the Vitamin C Contents of Some Leafy Vegetables Sold in Lokoja, Nigeria. Journal of Science, 5(1), 472–482
- Helmenstine, A.M. (2020). “Vitamin C Determination by Iodine Titration”. https://thoughtco.com/vitamin-c-determination-by-iodine-titration-606322.
- Samuel, F.O., Ayoola P. B., and Ejoh, S.I. (2020). Nutrient, Antinutrient and Sensory Evaluation of Corchorus Olitorius Fruit. Ife Journal of Agriculture, 32 (1), 13-20.
- Soto, V. C., González, R. E., and Galmarini, C. R. (2021). Bioactive compounds in vegetables, Is there consistency in the published information? A systematic review. Journal of Horticultural Science and Biotechnology, 96 (5), 570-587.
- D’elia, L., Obreja, G., Ciobanu, A., Breda, J., Jewell, J., and Cappuccio, F. P. (2019). Sodium, Potassium and Iodine Intake, in A National Adult Population Sample of the Republic of Moldova. Nutrients, 11(12), 1–16. https://doi.org/10.3390/nu11122896
- Palmer, B. F., and Clegg, D. J. (2019). Physiology and Pathophysiology of Potassium Homeostasis: Core Curriculum 2019. American Journal of Kidney Diseases, 74(5), 682–695.
- Fabbri, A. D., and Crosby, G. A. (2016). A Review of the Impact of Preparation and Cooking on the Nutritional Quality of Vegetables and Legumes. International Journal of Gastronomy and Food Science, 3: 2-11.
- Schlueter, A. K. and Johnston, C. S. (2011).Vitamin C: Overview and Update. Journal of Evidence-Based Complementary & Alternative Medicine, 16(1) 49-57.
- Ogunlesi, M., Okiei, W., Azeez, L., Obakachi, V., Osunsanmi, M., and Nkenchor, G. (2010). Vitamin C Contents of Tropical Vegetables and Foods Determined by Voltammetric and Titrimetric Methods and Their Relevance to the Medicinal Uses of the Plants. Int. J. Electrochem. Sci., 5, 105 – 115.