1.0 Introduction
Malaria infection is a public health disease that, threatened the lives of one-third of the world’s population, particularly in Africa (88 %), Asia (10 %), and Central and South America (2 %) [1]. Children under 5 years old and pregnant women with potentially compromised immune systems are most vulnerable to malaria infection and dominated a larger proportion of malaria-related death worldwide [2].
Currently, there are about 214 million cases, one million deaths annually are due to malaria, most especially in children of Sub-Sahara Africa [3].
Inflammation is a complex biological response of vascular tissues to harmful stimuli such as pathogens, damaged cells or irritants [4,5]. In the absence of inflammation, wounds and infections would never heal and the progressive destruction of the tissues would compromise the survival of the organism [5,6]. Although it is a defence mechanism, loss of homeostatic control of this process has been implicated in the aetiology and progression of certain diseases including cancers and diabetes [6-8].
Pain refers to the subjective, unpleasant sensation that accompanies damage or near damage to tissues. Though it can also occur in the absence of such damage [5]. Chemicals released locally as a result of cell injury either produces pain by direct stimulation or by stimulation of nerve endings responsible for the mediation of pain [5,9].
Several steroidal and non-steroidal anti-inflammatory drugs (NSAIDs), and immuno-suppressant drugs have been developed for the management of pain and inflammatory conditions [10]. However, these drugs although proven effective, were often associated with severe adverse side effects on the gastrointestinal, cardiovascular, bronchus, and hepatic system, and have limited their use [5,11]. Thus, the search for new drugs alternative with little or no side effects has led to the current trend of research on medicines of plant origin [12].
Natural products particularly from plants have been used in healthcare for the treatment of several kinds of human diseases [13-15]. They are considered to be effective and produce least or no adverse effects in clinical trials when compared to conventional synthetic drugs [16,17]. Medicinal plants serve as a source of effective therapeutic agents which have been explored as a backbone for the development of some conventional drugs used in the clinics [18]. Several classes of bioactive phytochemicals including alkaloids, flavonoids and other natural compounds, have been implicated in the biological activities of medicinal plants [19].
Halea ciliata (Mitragyna ciliata Aubrev. Et Pellegr also known as Abura leaves are common among the Yoruba groups of Nigeria for wrapping Kola nuts [20]. It is commonly used in traditional medicine for the treatment of inflammation, headache, malaria hypertension, gonorrhoea, rheumatism, and broncho-pulmonary diseases [21]. Several classes of secondary metabolites produced by Halea ciliata are effective in the wound healing process and have anti-microbial properties [22]. This study focuses on the evaluation of the anti-malarial, anti-inflammatory and analgesic activities in different animal models of extract from the leaf of Halea ciliata
2.0 Materials and Methods
2.1 Collection of plant material
The leaf of Halea Ciliata was collected from Ore, Odigbo Local Govt Area, Ondo State, Nigeria. The plant was authenticated by a botanist at Federal Polytechnic Ile-Oluji, Ondo State, Nigeria The Halea Ciliata was rinsed under clean running water, air-dried, and pulverized. The powdered sample was stored in an airtight container until it is ready for use.
2.2 Experimental animals
Swiss adult albino rats and mice were obtained from the animal house of the Federal Polytechnic Ile-Oluji. Ondo State, Nigeria. The animals were housed under standard laboratory conditions (Temperature 27 ± 2°C; 70% relative humidity; 12 h daylight/night cycle) and had free access to commercial feed pellets and water [12]. The animal study was approved and conducted in strict compliance with the principles governing the use of laboratory animals as laid out by the Federal Polytechnic Ile-Oluji. Ondo State Committee on Ethics for Medical and Scientific Research as contained in the Animal Care Guidelines and Protocol Review of National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1985).
2.3 Experimental parasite
The Plasmodium berghei NK65 chloroquine-sensitive strain was obtained from the National Institute of Pharmaceutical Research and Development (NIPRD) Abuja, Nigeria and maintained in the laboratory by serial passage in mice.
2.4 Plant extraction
The pulverized Halea Ciliata was extracted with methanol using a reflux extractor. Five hundred grams (500 g) of pulverized Halea Ciliata was extracted with methanol (2.5 litres) using a reflux extractor. The crude extraction yielded 7.35 % of the extract, which was concentrated, air-dried and stored in a refrigerator at 4 ˚C.
2.5 Phytochemical analysis
The crude methanol extract of Halea Ciliata was evaluated for the presence of major phytochemicals including alkaloids, flavonoids, phenols, tannins, glycosides, terpenes, saponins, steroids, phenols, and anthraquinones. [23-25].
2.6 Acute toxicity
The acute oral toxicity of the crude methanol extract of Halea Ciliata was evaluated in albino rats using Lorke’s method [26], as reported by Muhammad et al. [27].
2.7 Antimalarial screening
The antiplasmodial effect of the crude methanol extract of Halea Ciliata was evaluated using the four days suppressive test according to the established protocol [28]. Albino mice were inoculated with 0.2 ml (1×107) of Plasmodium berghei infected blood from donor mice. Thereafter, the mice were divided into 4 groups (n=5). Groups 1 and 2 were treated with 200 and 400 mg/kg of the crude methanol extract of Halea Ciliata respectively, while groups 3 and 4 were treated with 5 mg/kg chloroquine and 2.0 ml/kg of normal saline respectively for 5 days. The parasitaemia was monitored using the thin blood film and the percentage of parasitemia was determined as described by Ndako et al. [12].
2.8 Anti-inflammatory screening
The anti-inflammatory activity was tested using the egg albumin induced paw oedema in rats [29]. Oedema was induced by the injection of 0.01 ml egg albumin into the sub-planter surface on the right hind paw 30 min after administering of the crude methanol extract of Halea Ciliata (200 and 400 mg/kg), acetylsalicylic acid (ASA) (150 mg/kg) and normal saline (20 ml/kg). The hind paw was monitored using a digital vernier calliper at 15 min intervals for a period of 1 h, and the percentage inhibition of oedema was calculated as described by Ndako et al. [12].
2.9 Analgesic screening
The crude methanol extract of Halea Ciliata was evaluated for its analgesic effects using the hot plate method. The experimental grouping was as described above. The rats were placed on a hot plate maintained at 55 °C within the restrainer as described by Menendez et al. [30]. The reaction time (time taken for the rats to react to the thermal pain by licking their paws or jumping) was recorded at 15 min intervals for 60 min as described by Ndako et al. [12].
2.10 Statistical analysis
All experiments were conducted with at least three replicates. Analyses were conducted using statistical package for social science (SPSS) version 16 and presented as means ± standard error of the mean. One-way analysis of variance (ANOVA) at p< 0.05 were used for comparing the significant differences between treatment groups.
3.0 Results and Discussions
3.1 Phytochemical compositions of the crude methanol extract of Halea Ciliata
Qualitative phytochemical analysis of the crude methanol extract of Halea Ciliata revealed the presence of alkaloid, tannin, flavonoid, glycoside, steroid, terpenoid and saponin (Table 1) Recently, much attention has been focused on using plants as an alternative therapy for the treatment of various ailment. Phytochemicals are bioactive plant compounds that are responsible for various biological activities and toxicological virtue [31]. The presence of alkaloid, tannin, flavonoid, glycoside, steroid, terpenoid and saponin justified the traditional usage of Halea Ciliata for the treatment of several diseases. These flavonoids, alkaloids, saponins, tannins, and phenols identified in the extract have been reported for antioxidant, anti-diabetic, anti-cancer, anti-microbial, anti-parasitic, anti-hypertensive, analgesic anti-inflammatory and several other biological activities [32-35].
Table 1: Qualitative phytochemical composition of crude methanol extract of Halea Ciliata
| Test | Results |
| Alkaloid | + |
| Tannin | + |
| Flavonoid | + |
| Glycoside | + |
| Steroid | + |
| Terpenoid | + |
| Phenol | – |
| Saponin | + |
3.2 Acute toxicity and LD50 of the crude methanol extract of Halea Ciliata
Though medicinal plants are widely accepted and used in the treatment of many diseases, their toxicity must not be ignored [36]. Studies have also linked the toxicity of various plants to the presence of phytochemicals [31,32,37]. It is, therefore, become relevant to access the safety of Halea ciliata. Acute toxicity is the adverse effects that occur within 24 hours of ingesting a single dose of substance[38]. The crude methanol extract of Halea Ciliata had LD50 greater than 5000 mg/kg BW in the acute oral toxicity test (Table 2). During the 24 h of extract administrations as well as the 2 weeks’ observation period, none of the rats exhibited weight loss and no deterioration in health or mortality were observed in rats treated with the crude extract up to the dose of 5000 mg/kg, indicating that the LD50 is greater than 5000 mg/kg in rats (Table 2). Results of the present study suggested that the crude extract of Halea Ciliata exhibited a high degree of safety with LD50 greater than 5 kg/BW. Thus can be categorized as non-toxic extract (Class 5) in accordance with the guideline of the Organization for Economic Cooperation and Development (OCED) on acute oral toxicity testing based on LD50
Table 2: Acute toxicity profile of the crude methanol extract of Halea Ciliata
| Dose (mg/kg) | Mortality | |
| PHASE 1 | 10 | 0/3 |
| 100 | 0/3 | |
| 1000 | 0/3 | |
| PHASE 2 | 1600 | 0/1 |
| 2900 | 0/1 | |
| 5000 | 0/1 | |
| Normal Saline | 20ml/kgbw | 0/3 |
3.3 Anti-malaria activities of the crude methanol extract of Halea Ciliata in Plasmodium berghei infected mice
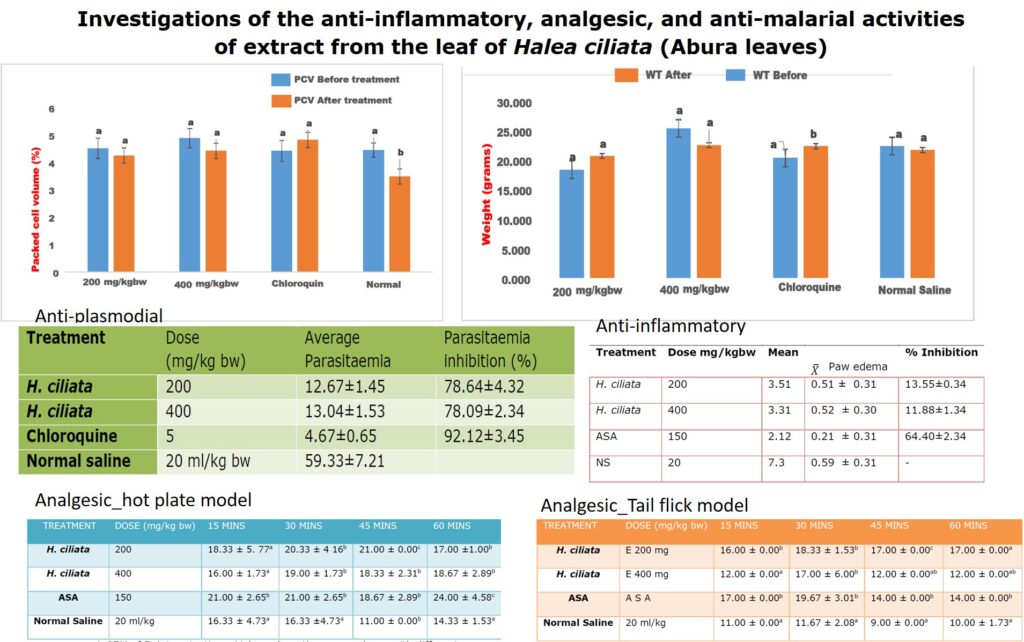
The crude methanol extract of Halea Ciliata demonstrated significant anti-plasmodial activities by suppressing the multiplication of the Plasmodium berghei parasite in the infected mice in a dose-independent manner (Table 3). The crude extract demonstrated 78.64±4.32 % and 78.09±2.34% inhibition of the Plasmodium berghei parasite, while chloroquine, the standard drug demonstrated the highest suppression of the parasite replication (92.12±3.45 %) when compared with the extract-treated group (Table 3). Interestingly, the extract also prevented the parasite induces loss of packed cell volume (PCV, figure 1), and body weight gain (figure 2), when compared with the infected non, treated control (Table 6). Halea ciliata is a traditional herb, which has been used traditionally for the treatment of some inflammatory and infectious diseases in Africa [39]. Collectively this finding revealed that the crude methanol extract of Halea Ciliata suppress the replication of plasmodium berghei infection and prolonged the survival days of the animal. Hence could serve as a template for the development of novel anti-malaria therapy
Table 3: In vivo anti-plasmodial activities of the crude methanol extract of Halea Ciliata in Plasmodium berghei infected mice
| Treatment | Dose (mg/kg bw) | Average Parasitaemia | Parasitaemia inhibition (%) |
| H. ciliata | 200 | 12.67±1.45 | 78.64±4.32 |
| H. ciliata | 400 | 13.04±1.53 | 78.09±2.34 |
| Chloroquine | 5 | 4.67±0.65 | 92.12±3.45 |
| Normal saline | 20 ml/kg bw | 59.33±7.21 |
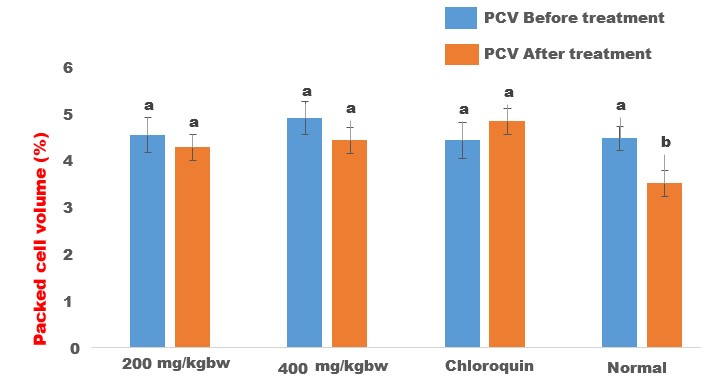
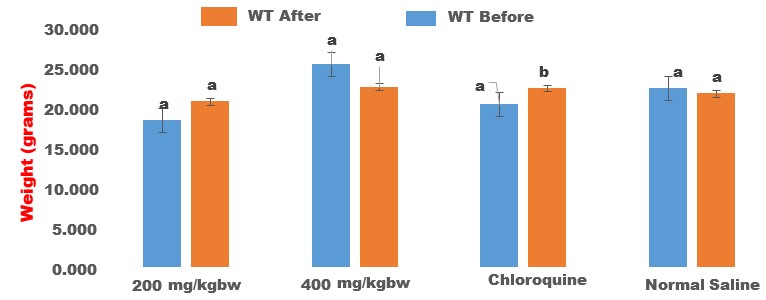
Figure 2: Effect of crude methanol extract of Halea ciliata on body weight of Plasmodium berghei infected mice. Bars followed by different superscript (p>0.05) alphabet are not significantly different
3.4 Anti-inflammatory and analgesic activities of the crude methanol extract of Halea ciliata
The crude methanol extract of Halea ciliata demonstrated significant anti-inflammatory activities in egg albumin induced paw oedema (Table 4). The extract significantly (p<0.00) suppressed the egg albumin induce rat paw oedema in a dose-independent manner. The percentage inhibition of the paw oedema was 13.55±0.34, and 11.88±1.34 at 200 and 400 mg/kg respectively, while the standard drug caused 64.40±2.34 inhibition of paw oedema when compared to the non-treated controls.
In the egg albumin-induced paw oedema model, the anti-inflammatory activities of crude methanol extract of Halea ciliata were evident from 0 to 60 minutes of the treatment, indicating that both the extract is effective against the early and late stage of acute inflammation. Flavonoids are well-known anti-oxidative agents, hence, the anti-inflammatory activity observed in this study could be attributed to the inhibition of PG synthesis, membrane stabilization, and antioxidant activities as they play key roles in countering the establishment of acute and chronic inflammation [40]. These observations provide support for the traditional use of Halea ciliata in the treatment of inflammation and infectious diseases.
Furthermore, when compared with the non-treated controls, the crude methanol extract of Halea ciliata demonstrated significant analgesic activities in both the hot plate (Table 5) and tail-flick (Table 6) models. The use of the hot plate model of thermal stimulus to induce pain is a widely used technique of evaluating the centrally acting analgesic drugs [30] and hence we used this model to evaluate the anti-nociceptive activity of Halea ciliata leaves. The results of the present study indicated that the crude methanol extract of Halea ciliata demonstrated a dose-independent anti-nociceptive effect against thermal-induced pain. The analgesic effect of the crude methanol extract of Halea ciliata increases with time suggesting higher pain suppression at the late phase than the early phase and could be associated with the pronounced inhibition of PG synthesis and membrane stabilization activities of the Halea ciliata [40].
Table 4: Anti-inflammatory effect of the crude methanol extract of Halea ciliata
| Treatment | Dose mg/kgbw | Mean | | % Inhibition |
| H. ciliata | 200 | 3.51 | 0.51 ± 0.31 | 13.55±0.34 |
| H. ciliata | 400 | 3.31 | 0.52 ± 0.30 | 11.88±1.34 |
| ASA | 150 | 2.12 | 0.21 ± 0.31 | 64.40±2.34 |
| NS | 20 | 7.3 | 0.59 ± 0.31 | – |
Values are mean ± SEM of 5 determinations. Values along the same column with different superscripts are significantly different. (p< 0.05). ASA= Acetyl Salicylic Acid. NS= Normal Saline
Table 5: Analgesic Activity of effect of the crude methanol extract of Halea ciliata in hot plate test
| TREATMENT | DOSE (mg/kg bw) | 15 MINS | 30 MINS | 45 MINS | 60 MINS |
| H. ciliata | 200 | 18.33 ± 5. 77a | 20.33 ± 4 16b | 21.00 ± 0.00c | 17.00 ±1.00b |
| H. ciliata | 400 | 16.00 ± 1.73a | 19.00 ± 1.73b | 18.33 ± 2.31b | 18.67 ± 2.89b |
| ASA | 150 | 21.00 ± 2.65b | 21.00 ± 2.65b | 18.67 ± 2.89b | 24.00 ± 4.58c |
| Normal Saline | 20 ml/kg | 16.33 ± 4.73a | 16.33 ±4.73a | 11.00 ± 0.00b | 14.33 ± 1.53a |
Values are mean ± SEM of 5 determinations. Values along the same column with different superscripts are significantly different. (p< 0.05). ASA= Acetyl Salicylic Acid; NS= Normal Saline
Table 6: Analgesic Activity of effect of the crude methanol extract of Halea ciliata in Tail flick test
| TREATMENT | DOSE (mg/kg bw) | 15 MINS | 30 MINS | 45 MINS | 60 MINS |
| H. ciliata | E 200 mg | 16.00 ± 0.00b | 18.33 ± 1.53b | 17.00 ± 0.00c | 17.00 ± 0.00a |
| H. ciliata | E 400 mg | 12.00 ± 0.00a | 17.00 ± 6.00b | 12.00 ± 0.00ab | 12.00 ± 0.00ab |
| ASA | A S A | 17.00 ± 0.00b | 19.67 ± 3.01b | 14.00 ± 0.00b | 14.00 ± 0.00b |
| Normal Saline | 20 ml/kg | 11.00 ± 0.00a | 11.67 ± 2.08a | 9.00 ± 0.00a | 10.00 ± 1.73a |
Values are mean ± SEM of 3 determinations. Values along the same column with different superscripts are significantly different. (p< 0.05). ASA= Acetyl Salicylic Acid, NS= Normal Saline
4.0 Conclusion
The results provide scientific evidence that crude methanol extract of Halea ciliata has potent anti-malaria, anti-inflammatory and anti-nociceptive activities, and justify the traditional use of the plant for the management of inflammation and malaria and associated diseases.
Authors’ contributions: All authors participate in research design, data collections, analysis and writing of the manuscript. All authors read and approved the final manuscript.
Funding: This work is supported by the TETFUND Institution-Based Research Intervention (IBRI) Fund of the Federal Polytechnic Ile-Oluji. Ondo State, Nigeria to Godwin Gabriel Omula. Reference No: TET/DR&D/CE/POLY/ILE-OLUJI/IBR/2019/.
Acknowledgements: The authors acknowledge the Laboratory Technologist of the Department of Science Laboratory Technology, Federal Polytechnic Ile-Oluji, Ondo State, Nigeria for their technical assistance
Conflicts of interest: The authors declare no conflicts of interest.
References
1. WHO. World Health Organization. World malaria report 2020: 20 years of global progress and challenges. Availabe online: (accessed on
2. Crawley, J. Reducing the burden of anemia in infants and young children in malaria-endemic countries of Africa: from evidence to action. The American journal of tropical medicine and hygiene 2004, 71, 25-34.
3. Kogan, F. Malaria Burden. In Remote Sensing for Malaria, Springer: 2020; pp. 15-41.
4. Libby, P.; Ridker, P.M.; Hansson, G.K.; Atherothrombosis, L.T.N.o. Inflammation in atherosclerosis: from pathophysiology to practice. Journal of the American college of cardiology 2009, 54, 2129-2138.
5. Egesie, U.; Chima, K.; Galam, N. Anti-inflammatory and analgesic effects of aqueous extract of Aloe Vera (Aloe barbadensis) in rats. African Journal of Biomedical Research 2011, 14, 209-212.
6. Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204.
7. Onikanni, A.S.; Lawal, B.; Olusola, A.O.; Olugbodi, J.O.; Sani, S.; Ajiboye, B.O.; Ilesanmi, O.B.; Alqarni, M.; Mostafa-Hedeab, G.; Obaidullah, A.J., et al. Sterculia tragacantha Lindl Leaf Extract Ameliorates STZ-Induced Diabetes, Oxidative Stress, Inflammation and Neuronal Impairment. J Inflamm Res 2021, 14, 6749-6764, doi:10.2147/jir.s319673.
8. Onikanni, A.S.; Lawal, B.; Oyinloye, B.E.; Mostafa-Hedeab, G.; Alorabi, M.; Cavalu, S.; Olusola, A.O.; Wang, C.-H.; Batiha, G.E.-S. Therapeutic efficacy of Clompanus pubescens leaves fractions via downregulation of neuronal cholinesterases/Na+-K+ATPase/IL-1 β, and improving the neurocognitive and antioxidants status of streptozotocin-induced diabetic rats. Biomedicine & Pharmacotherapy 2022, 148, 112730, doi:https://doi.org/10.1016/j.biopha.2022.112730.
9. Clarke, C. Neurological diseases in Parveen, K. and Michael, C. Clinical Medicine. W. B Saunders 2001, 1035-1036.
10. Rainsford, K. Anti-inflammatory drugs in the 21st century. Inflammation in the pathogenesis of chronic diseases 2007, 3-27.
11. Straus, W.L.; Ofman, J.J. Gastrointestinal toxicity associated with nonsteroidal anti-inflammatory drugs: epidemiologic and economic issues. Gastroenterology Clinics 2001, 30, 895-920.
12. Ndako, M.; Jigam, A.A.; Kabiru, A.Y.; Umar, S.I.; Lawal, B. Polar extracts from Gymnosporia senegalensis (syn. Maytenus senegalensis) root bark, its effects on nociception, edema, and malarial infection. Phytomedicine Plus 2021, 1, 100113, doi:https://doi.org/10.1016/j.phyplu.2021.100113.
13. Lawal, B.; Shittu, O.K.; Oibiokpa, F.I.; Berinyuy, E.B.; Mohammed, H. African natural products with potential antioxidants and hepatoprotectives properties: a review. Clinical Phytoscience 2017, 2, 1-66.
14. Lawal, B.; Shittu, O.K.; Kabiru, A.Y.; Jigam, A.A.; Umar, M.B.; Berinyuy, E.B.; Alozieuwa, B.U. Potential antimalarials from African natural products: A reviw. Journal of intercultural ethnopharmacology 2015, 4, 318.
15. Iwu, M.M. African medicinal plants; CRC Press, Maryland: 1993.
16. Salleh, N.H.; Zulkipli, I.N.; Mohd Yasin, H.; Ja’afar, F.; Ahmad, N.; Wan Ahmad, W.A.N.; Ahmad, S.R. Systematic Review of Medicinal Plants Used for Treatment of Diabetes in Human Clinical Trials: An ASEAN Perspective. Evid Based Complement Alternat Med 2021, 2021, 5570939, doi:10.1155/2021/5570939.
17. Spiller, H.; Sawyer, T. Toxicology of oral antidiabetic medication. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists 2006, 63, 929-938, doi:10.2146/ajhp050500.
18. Glynn, J.; Bhikha, R. Herbal products and conventional drugs–an uneasy alliance. Bangladesh Journal of Medical Science 2019, 18, 24-29.
19. Howes, M.-J.R.; Simmonds, M.S. The role of phytochemicals as micronutrients in health and disease. Current Opinion in Clinical Nutrition & Metabolic Care 2014, 17, 558-566.
20. ADEBISI, A.; LADIPO, D.; ADEWALE, J.; ADEKUNLE, I.; OKORO, M.; BUSARI, O.; OYEBAMIJI, T.; OMILABU, S. THE ECONOMIC POTENTIALS OF GARCINIA KOLANUT PRODUCTION IN J4 OMO FOREST RESERVE, SOUTHWEST NIGERIA. Journal of Sustainable Development 2017, 11, 35-41.
21. Dongmo, A.; Kamanyi, A.; Dzikouk, G.; Nkeh, B.C.-A.; Tan, P.; Nguelefack, T.; Nole, T.; Bopelet, M.; Wagner, H. Anti-inflammatory and analgesic properties of the stem bark extract of Mitragyna ciliata (Rubiaceae) Aubrév. & Pellegr. Journal of Ethnopharmacology 2003, 84, 17-21.
22. Altuner, E.; Cetin, B.; Cokmus, C. Antimicrobial screening of some Mosses collected from Anatolia. Pharmacognosy Magazine 2010, 6, 56.
23. Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High Molecular Weight Plant Polyphenolics (Tannins) as Biological Antioxidants. J Agric Food Chem 1998, 46, 1887-1892, doi:10.1021/jf970975b.
24. Trease, G. Trease and Evans. Pharmacognosy, A Physician’s Guide to Herbal Medicine 1989, 13, 912.
25. Makkar, H.P.S.; Blümmel, M.; Borowy, N.K.; Becker, K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. Journal of the Science of Food and Agriculture 1993, 61, 161-165, doi:10.1002/jsfa.2740610205.
26. Lorke, D. A new approach to practical acute toxicity testing. Archives of toxicology 1983, 54, 275-287.
27. Kifayatullah, M.; Mustafa, M.S.; Sengupta, P.; Sarker, M.M.R.; Das, A.; Das, S.K. Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.) Merr. in BALB/c mice. Journal of Acute Disease 2015, 4, 309-315, doi:https://doi.org/10.1016/j.joad.2015.06.010.
28. Peters, W. Chemotherapy and drug resistance in malaria. Chemotherapy and drug resistance in malaria. 1970.
29. Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proceedings of the society for experimental biology and medicine 1962, 111, 544-547.
30. Menéndez, L.; Lastra, A.; Hidalgo, A.n.; Baamonde, A. Unilateral hot plate test: a simple and sensitive method for detecting central and peripheral hyperalgesia in mice. Journal of neuroscience methods 2002, 113, 91-97.
31. Kuete, V. Toxicological survey of African medicinal plants. 2014.
32. Guldiken, B.; Ozkan, G.; Catalkaya, G.; Ceylan, F.D.; Ekin Yalcinkaya, I.; Capanoglu, E. Phytochemicals of herbs and spices: Health versus toxicological effects. Food and Chemical Toxicology 2018, 119, 37-49, doi:https://doi.org/10.1016/j.fct.2018.05.050.
33. Harsha, N.; Sridevi, V.; Lakshmi, M.; Rani, K.; Vani, N.D.S. Phytochemical analysis of some selected spices. Int J Innov Res Sci Eng Technol 2013, 2, 6618-6621.
34. Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutrition journal 2010, 9, 1-11.
35. Arora, S.; Kaur, K.; Kaur, S. Indian medicinal plants as a reservoir of protective phytochemicals. Teratogenesis, carcinogenesis, and mutagenesis 2003, 23, 295-300.
36. Raji, R.O.; Muhammad, H.L.; Abubakar, A.; Maikai, S.S.; Raji, H.F. Acute and sub-acute toxicity profile of crude extract and fractions of Gymnema sylvestre. Clinical Phytoscience 2021, 7, 1-9.
37. Lu, B.; Li, M.; Yin, R. Phytochemical content, health benefits, and toxicology of common edible flowers: a review (2000–2015). Critical Reviews in Food Science and Nutrition 2016, 56, S130-S148.
38. Amos, T.; Bashir, L.; Saba, S.; Saba, M.; Mohammed, B.; Abdulsalam, I.; Josiah, J. Phytochemicals and acute toxicity profile of aqueous and methanolic extracts of Crateva adansonii leaves in Swiss albino rats. Asian J Biochem 2015, 10, 173-179.
39. Da Silva, G.; Serrano, R.; Silva, O. Maytenus heterophylla and Maytenus senegalensis, two traditional herbal medicines. Journal of Natural Science, Biology, and Medicine 2011, 2, 59.
40. Vane, J.R.; Botting, R.M. Mechanism of action of nonsteroidal anti-inflammatory drugs. The American journal of medicine 1998, 104, 2S-8S.