1.0 Introduction
Diatomic oxygen is an unavoidable product of aerobic metabolism which is formed from the incomplete reduction or oxidation of molecules during metabolism generating reactive oxygen species (ROS) [1]. ROS plays a dual role as both toxic and beneficial compounds. At moderate or low concentration, ROS mediates essential roles in the body, among other things: immune function, cell signaling, redox regulation, and apoptosis[2]. However, at high levels, they become harmful to cell [3].Enhanced generation of ROS in the cell may induce oxidative stress, and this has been implicated in the pathogenesis of many chronic and degenerative disease[4,5]. Antioxidant systems in living organisms are capable of scavenging and stabilizing ROS by donating additional electrons, delaying or inhibiting the oxidation of substrates [6]. They are produced in situ or sourced externally from food and supplements [7]. Studies have shown that the natural human antioxidant defenses are not always sufficient to maintain the proper ROS balance, and some normal biological processes can become detrimental when it persists long term [6]. This has led to the search for exogenous (synthetic) antioxidants by man to ameliorate oxidative stress in the body. Butylated hydroxytoluene (BHT) is a synthetic antioxidant allowed for use in fat and oils, heat treated foods, frying oil, frying fats, and in beef, poultry, and sheep fats. Inadvertent exposure to BHT in animals has been shown to induce renal and liver toxicities, reduce humoral immune response in animals [8]
Recent research studies reveal the need to replace synthetic antioxidants such as BHT with natural antioxidants such as plants and herbs [9]. In this direction, natural antioxidants have been used in place of synthetic ones because of their broad spectrum of applications in the prevention and treatment of diseases and broad safety margin; and this has influenced food manufacturers, pharmaceutical industries, and many investigators [10-12]. A. cepa (onions) is the most widely cultivated species of the genus Allium. Externally, fresh onion juice is used to prevent bacterial and fungal infections [13], remove warts [14], stimulate hair growth [15], and reduce unwanted skin blemishes[15-17]. Onion contains a rich blend of S-methyl cysteine sulfoxide, flavonoids such as quercetin and organosulfur compounds such as allyl propyl disulphide [18]. Experimental reports have shown that these phytochemicals found in onions have antioxidant [19,20], anti-inflammatory, hypolipidemic [21], and antidiabetic effects [22]. M. myristica (African nutmeg) is an easily sourced condiment used to prepare different delicacies because of its aromatic flavour [23]. Traditionally, it has been used to heal sores caused by guinea worms [24], constipation [25], stomach ache [26], headache [27,28]as well as to stop intra-uterine bleeding in women after childbirth [29]. The oil from the seed is used in relieving the discomfort of gas in the digestive tract, for making perfume and soap scents [27,30]. The bark is used in the treatment of stomach aches, febrile pains[31], eye diseases [32], while the root is munched to mitigate toothaches, arthritis [33,34]and also in the management of anaemia [27,33], haemorrhoids as well as sexual weakness [30,32]. Studies have revealed that M. myristica possesses antioxidant [24,28], hypolipidemic[33], anti-sickling, antimicrobial, [25,26] as well as anthelmintic activities [27]. A. sativum (garlic) is the most widely consumed bulb after onion [35]. Traditionally, it has been used as a flavouring agent [13], seasoning in cooking [36], and in medicine to manage fever[37], headache [38], cholera, and dysentery[35]. A component of garlic, S-methyl cysteine sulfoxide, has been shown to reduce both blood cholesterol and the severity of atherosclerosis [39], stroke [40], coronary thrombosis, platelet aggregation, as well as infections and vascular disorder [17]. The study aimed to investigate the phytochemical contents and antioxidant capacity of A. cepa, A. sativum, and M. myristica through in vitro and in vivo methods. The outcome of this study will serve as a blueprint for the branding of these condiments as natural antioxidants in the food industry.
2.0 Material and methods
2.1 Chemicals
Trichloroacetic acid (TCA), thiobarbituric acid (TBA), butylated hydroxyl toluene (BHT), hydrogen peroxide, dichromate, xylenol orange, sorbitol, were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). All other reagents were of analytical grade.
2.2 Plant collection and preparation
A. cepa (bulb), A. sativum (bulb), and M. myristica (seed) were purchased from Nyanya market in Abuja, Nigeria. They were peeled, washed, and allowed to air dry. The dried samples were coarsely minced and blended to a fine powder. Exactly 300 g of each of the samples were soaked in 700 ml of distilled water for 48 hours and then filtered using Whatman filter paper No1. The filtrate was evaporated to dryness in a water bath at 100°C and the preparations served as stock and were stored at 4°C.
2.3 Phytochemical screening
Phytochemical screening was carried out with the aqueous extract of A. cepa, A. sativum, and M. myristica for the detection of various phytochemicals. The extracts were tested for the presence of tannins, saponins, glycosides, alkaloids, flavonoids and phenolic compounds using the standard procedures described by Trease and Evans [41].
2.4 Reducing power assay
The reducing power of the extracts were determined by a method described by Oyaizu [42]. Different concentrations of extracts in 1 mL of distilled water were mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferrocyanide. The mixtures were incubated at 50°C for 20 min. Aliquots 2.5 mL of 10% trichloroacetic acid were added to the mixtures and centrifuged at 3000 rpm for 10 min, the supernatant of the solution (2.5 mL) was added to 2.5 mL of distilled water and 0.5 mL of 0.1% FeCl3. The absorbance was read at 700 nm by UV-Spectrophotometer.
2.5 In vivo antioxidant assay
A total of 45 male Wistar rats (185±10 g) raised in the animal house of Bingham University were used for the experiment. Animals were housed in polypropylene cages and kept at standard laboratory condition (at 24 ± 2 °C under 12:12 h of light and dark cycle). The research was carried out in accordance to the ethical rules on animal experimentation approved by the ethical committee of Bingham University. The animals had free access to a standard pellet diet and water ad libitum. After that, the animals were divided into five groups of 9 rats each, as described below:
Group I rats were administered with 0.5 mL/kg BW distilled water alone
Group II rats were administered with 100 mg/kg BW ascorbic acid
Group III rats were administered with 100 mg/kg BW A. cepa
Group IV rats were administered with 100 mg/kg BW M. myristica
Group V rats were administered with 100 mg/kg BW A. sativum
Three rats from each group were sacrificed on the 20th, 25th, and 30th days of oral administration through cervical dislocation. The animals were fed once daily with commercially formulated rat feed, and water was given ad libitum. The research was carried out in accordance to the ethical rules on animal experimentation approved by the ethical committee of Bingham University.
2.6 Preparation of liver and kidney homogenate
The liver and kidney were excised from the sacrificed Wistar rats the organs were weighed, kept in precold 0.1M phosphate buffer, pH 7.4 and 10% homogenate were prepared by homogenizing 10 g of each organ in the cold phosphate buffer at 4°C. The homogenate was centrifuged at 3000 rpm for 15 min, and the clear cell-free supernatant obtained was used for the study.
2.7 Determination of oxidative stress biomarkers
The supernatants of the liver and kidney tissues of control, ascorbic acid, A. cepa, A. sativum and M. myristica treated rats were collected for the estimation of catalase (CAT) activity using hydrogen peroxide as a substrate according to the method of Clairborne [43]. Hydrogen peroxide generation was assessed by the method of Wolff [44]. Lipid peroxidation was quantified as the amount of malondialdehyde (MDA) produced according to the method described by Varshney and Kale [45].
2.8 Statistical analysis
The experiments were conducted on the 20th ,25th and 30th day of administration, all determinations were performed in triplicates, and results were expressed as Mean ± SEM. Statistical analysis was performed by one-way analysis of variance (ANOVA) with GraphPad Prism statistical software package, version 9. Statistically significant differences were set at values of p<0.05.
3.0 Results
3.1 Phytochemical composition
The phytochemical constituents of aqueous extracts of the selected condiments consisting of A. cepa, A. sativum, and M. myristica, were analyzed qualitatively, as shown in Table 1. The spices are replete in the following phytochemicals at varying intensity: flavonoid, tannin, saponin, cardiac glycoside, terpene, steroid, and phlobatannin. Alkaloid was present in A. cepa and A. sativum only.
Table 1. Phytochemical composition of aqueous extract of selected food condiments
| Phytochemicals | A. cepa | M. myristica | A. sativum |
| Alkaloid | ++ | – | + |
| Flavonoid | + | +++ | ++ |
| Tannin | + | + | +++ |
| Saponin | + | + | +++ |
| Cardiac glycoside | ++ | + | +++ |
| Terpene | ++ | ++ | ++ |
| Steroid | + | + | ++ |
| Phlobatannin | + | + | + |
-; Absent +; Mildly present, ++; Moderately present, +++; Abundantly present
3.2 In vitro antioxidant capacity
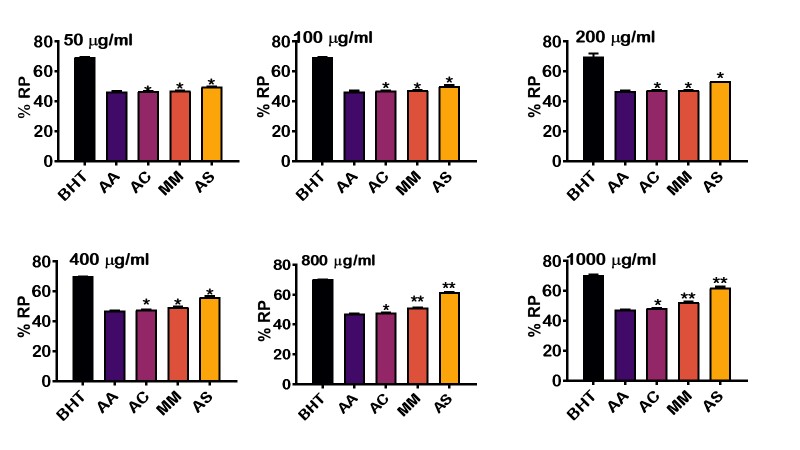
To investigate the antioxidant capacity of the crude extract of selected food condiments (A. cepa, A. sativum and M. myristica), the reducing capacity of these extracts were measured using reducing power (%) at various concentrations (50 –1000 µ/mg) and compared it with synthetic antioxidants; butylated hydroxytoluene and ascorbic acid (Figure1). The result revealed that the percentage reducing power slightly increased with concentrations, i.e., from 50 to 1000 µ/mg. BHT recorded the highest percentage reducing power at all levels. This is followed by A. sativum, M. myristica, A. cepa, and ascorbic acid. There is a significant difference (p<0.05) in the percentage reducing power between BHT and the selected condiments at various concentrations. However, there is no statistical difference in the percentage reducing capacity when compared to ascorbic acid. The results further revealed that at higher concentrations of 800 and 1000 µ/mg, the percentage reducing power of M. myristica and A. sativum increased significantly when compared to BHT and ascorbic acid.
3.3 Effect of selected food condiments on oxidative stress biomarkers
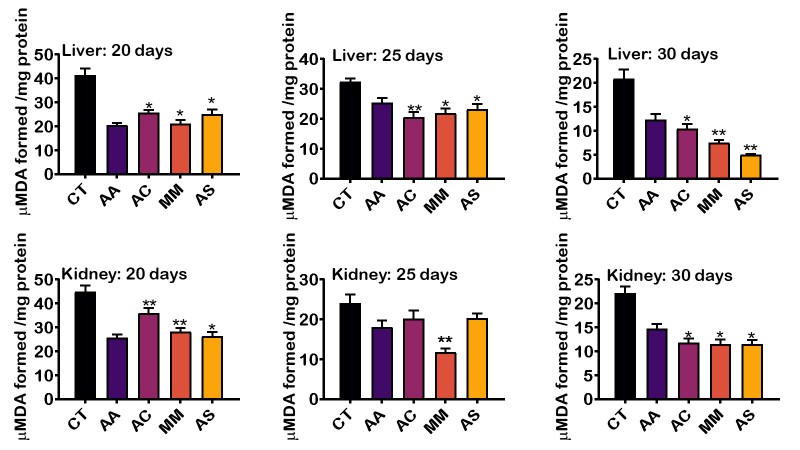
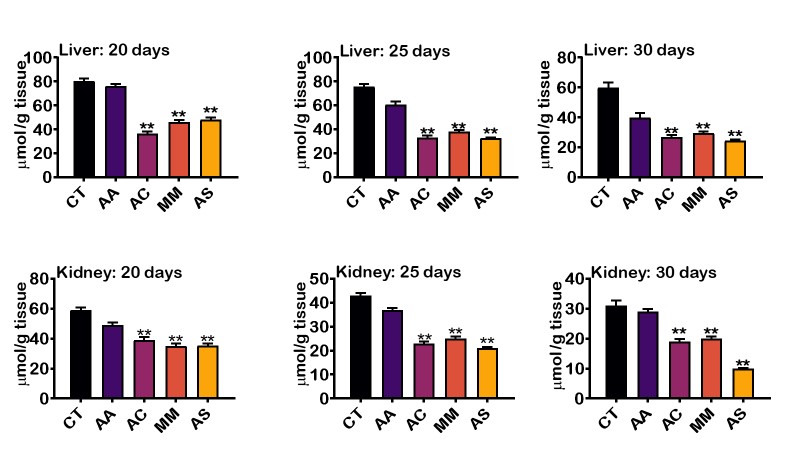
Figures 2 and 3 showed the level of MDA and H2O2 respectively formed by the extracts on the 20th,25th and 30th day of administration. The level of LPO decreased steadily with A. sativum having the least LPO level, followed by M. myristica, A. cepa, ascorbic acid and then control. Interestingly, there was marked significant difference in the free radical scavenging activity in the liver of rats treated with aqueous extract A. sativum and M, myristica when compared to the synthetic antioxidant supplement, ascorbic acid. In the kidney of animals treated with the aqueous extract of the selected food condiments, there was no significant difference in the level of LPO compared to ascorbic acid (Figure 2). Also, the level of H2O2 in the liver and kidney tissues was significantly decreased in day 20, day 25 and day 30 (Figure 3). The result showed that H2O2 in the liver was reduced considerably by A. cepa, M. myristica, and A. sativum in days 20, 25, and 30 when compared with the control group that received distilled water only and ascorbic acid. At day 20 and 25, A. cepa recorded the highest free radical scavenging activity, whereas, on day 30, the level of H2O2 was lowered by A. sativum. In the kidney, a similar observation was made. However, the levels of H2O2 in days 20, 25, and 30 decreased significantly in the groups treated with A. sativum followed by A. cepa and then M. myristica
3.5 Effect of some selected food condiments on enzymatic Antioxidants.
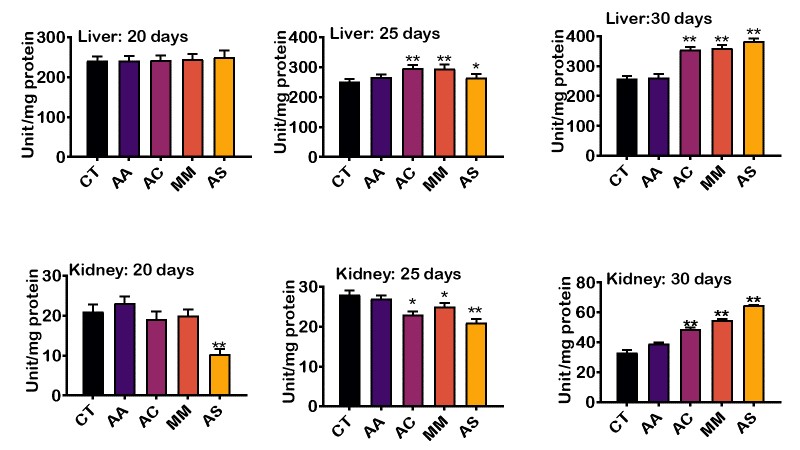
Activity of catalase was evaluated in the liver and kidney across the different groups, and the result is presented in Figure 4. The result revealed an increase in the activity of catalase in the liver and kidney catalase on the 20th, 25th, and 30th day. The liver catalase level at day 20 did not differ significantly when compared with the control and ascorbic acid. However, at days 25 and 30, the level of catalase significantly increased in rats treated with A. cepa, M. myristica, and A. sativum when compared to ascorbic acid and control groups. The kidney catalase levels at day 20 and 25 were, however, lower in groups treated with A. cepa, M.myristica, and A. sativum when compared to those treated with ascorbic acid. At day 30, catalase level in groups that received food condiments peaked significantly compared to the ascorbic acid and control groups
4.0 Discussion
Reactive oxygen species (ROS), such as hydroxyl radicals, superoxide radicals and hydrogen peroxide, are generated in living systems under favorable conditions [46]. Excess production of ROS can alter the homeostatic balance thereby initiating or exacerbating degenerative diseases in animals. However, endogenous antioxidants including superoxide dismutase (SOD), catalase, glutathione peroxidase, glutathione, and vitamins can prevent the onset of diseases by scavenging the excess ROS in the body [47]. Studies have shown that, our body does not need only endogenous antioxidants to fight against free radicals but also plant-derived antioxidants such as. vitamins A, C, E, polyphenols, flavonoids to effectively reduce the harmful effects of ROS [11]. To add to existing knowledge in the pharmacopeia, we investigated the in vitro and phytochemical constituents of selected food condiments (A. cepa, A. sativium, and M. myristica)as well as in vivo antioxidant activity in the liver and kidney of rats. Virtually all plants have one or more phytochemical residents in their leaves, stems, roots, fruits, and flowers [48]. From our present findings, the aqueous extracts of the selected condiments contain phytochemicals that contributed to their antioxidant capacity [18] and the pharmacological and biochemical actions of these phytochemicals have long been established. For instances, alkaloids possess analgesic, antispasmodic, antibacterial and antineoplastic effects [17]; flavonoids are potent antioxidants that protect the human body against free radicals and have potent anticancer activity [49]; saponins have been found to possess hypocholesterolemic, antitumor, antioxidant, antimutagenic and antibiotic properties [50]while tannins possess among other functions, antioxidant, anti-inflammatory, antiulcer and anticancer effects[32]. The phytoprotective activity of one of the condiments (M. myristica)against sodium arsenite-induced clastogenicity in Wistar rats has long been investigated[51], thus validating our findings that these phytochemicals may confer health benefits.
Ferric reducing antioxidant power is a protective antioxidant mechanism used to evaluate metal ions binding ability [52]. The reducing power is commonly related to the presence of efficacious molecules known as reductones that exhibits their antioxidant capacity by disrupting the free radical chain and donating hydrogen atom [53]. Ferric reducing assay evaluates the ability of antioxidant components to reduce Fe2+ / ferricyanide complex to its ferrous form, this reaction has a deep blue color [54]. From the present result, it was observed that the reducing property was highest in A. sativum, followed by A. cepa, and then M. myristica. The reducing power of these extracts increased with increase in the concentrations of plant extracts. This observation is line with earlier postulation that the ability of antioxidants to reduce Fe3+ to Fe2+ was found to increase as the concentration increased [54]. The reducing power of selected food condiments suggests that the extracts may have antioxidant capacity.
Reactive oxygen species react with all biological substances [40]; however, the most vulnerable ones are polyunsaturated fatty acids [55]. Free radicals are unpaired electrons that bounce around and destroy healthy cells, attacking the cell membranes, leading to DNA damage and mutation. They react with the cellular membrane lipids, causing peroxidation of polyunsaturated fatty acids, and causing further generation of free radicals [56]. Due to the electron-donating and antioxidant capacity of plant extracts, they can mop out excess free radicals and reduce the generation of malondialdehyde (a marker of lipid peroxidation) in the body, thereby restoring the redox rheostat [57]. In this investigation, the level of malondialdehyde is used as a marker of lipid peroxidation [58]. The time-dependent decrease in the level of malondialdehyde by the different food condiments suggests that they are richly available in endogenous antioxidants [35]. A. sativum depicted the highest level of reduction due to the active ingredients such as phenols and saponins, which have a direct effect on the metabolism of cytochrome P450 and glutathione S-transferase. This result is consistent with studies done by Kang [59] and also Park [60] which showed that the mechanism of antioxidative action of A. sativum might be involved with the enhancement of antioxidant enzyme activities.
Hydrogen peroxide is widely regarded as a cytotoxic agent whose level must be minimized by the action of antioxidants [19]. The result showed a time-dependents decrease in the hydrogen peroxide generation of the selected food condiments when compared to the control indicating that the spices can protect cells from oxidative damage occasioned by exposure to toxic hydrogen peroxide [61]. A. sativum showed the highest level of reduction, and this is because of the bioactive compounds in A. sativum, which are crucial in the protection of the liver and kidney. Allicin is a bioactive compound in A. sativum and has been shown to mediate anti-oxidative activity [62].
Catalase is an enzymatic antioxidant widely distributed in all animal tissue [63]. It carries out the detoxification of H2O2 into molecular oxygen and protects the tissues from highly reactive hydroxyl radicals [64]. Catalase is an inducible enzyme whose production can be stimulated. Therefore, the elevated activity of catalase may suggest that there is an induction of the enzyme by the extracts in the rats, which corresponds with earlier reports that catalase can be induced in experimental animals [40]. The time-dependent increase in the activity of catalase enzyme in this study could also mean the enhanced antioxidative capacity of the animals, which leads to the inactivation of lipid peroxides, hence a decreased level of malondialdehyde. A. sativum showed the highest increase in this study because it contains mainly diallyl disulfide and other sulfur compounds that protect the cells through influencing peroxide forms and oxidation-reduction.
5.0 Conclusion
The antioxidant activity of A. sativum, M. myristica and A. cepa were investigated in this study, and the outcomes showed that these food condiments are rich in antioxidants as they were able to reduce the level of ROS as well as increase the level of endogenous antioxidants. Interestingly, the in vitro antioxidant activity of the three food condiments was higher than that of ascorbic acid – a standard antioxidant agent. This suggests that the inclusion of these food condiments in our diet during food preparation may ameliorate oxidative stress and reduce the incidence of degenerative disease in animals. Although results from animal studies cannot be extrapolated directly to humans, further studies need to be done on the standard of dosage to minimize any adverse side effects.
Acknowledgements: All authors would like to thank the Laboratory staff of the Department of Biochemistry, Bingham University, Karu, Nasarawa State, Nigeria for their technical assistance.
Disclosure statement: The Authors declares that there is no conflict of interest.
Author Contributions: Project administration and Supervision: Dr. Janet Olubodi; Conceptualization: Olusola Ladeji. Data curation, analysis, investigation: Chioma Irozuru; Writing, review, and editing: Chioma Irozuru and Uche Okuu Arunsi.
Funding statement: This research was done without a specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. Taibur Rahman, I.H., M. M. Towhidul Islam, Hossain Uddin Shekhar. Oxidative stress and human health Advances in Bioscience and Biotechnology 2012, 3, 997-1019
2. Oyinloye, B.E.; Adenowo, A.F.; Osunsanmi, F.O.; Ogunyinka, B.I.; Nwozo, S.O.; Kappo, A.P. Aqueous extract of Monodora myristica ameliorates cadmium-induced hepatotoxicity in male rats. Springerplus 2016, 5, 641, doi:10.1186/s40064-016-2228-z.
3. Farombi, E.O.; Owoeye, O. Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int J Environ Res Public Health 2011, 8, 2533-2555, doi:10.3390/ijerph8062533.
4. Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ J 2012, 5, 9-19, doi:10.1097/WOX.0b013e3182439613.
5. Droge, W. Free radicals in the physiological control of cell function. Physiol Rev 2002, 82, 47-95, doi:10.1152/physrev.00018.2001.
6. Shao, H.B.; Chu, L.Y.; Lu, Z.H.; Kang, C.M. Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 2007, 4, 8-14, doi:10.7150/ijbs.4.8.
7. Olaiya, C.O.; Soetan, K.O.; Esan, A.M. The role of nutraceuticals, functional foods and value added food products in the prevention and treatment of chronic diseases. African Journal of Food Science 2016, 10, 185-193, doi:10.5897/ajfs2015.1402.
8. Rebecca S Lanigan, T.A.Y. Final Report on the Safety Assessment of EDTA, Calcium Disodium EDTA, Diammonium EDTA, Dipotassium EDTA, Disodium EDTA, TEA-EDTA, Tetrasodium EDTA, Tripotassium EDTA, Trisodium EDTA, HEDTA, and Trisodium HEDTA. International Journal of Toxicology 2016, 21, 95-142, doi:10.1080/10915810290096522.
9. Gopalakrishnan, A.; Tony Kong, A.N. Anticarcinogenesis by dietary phytochemicals: cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-kappa B and AP-1 in abnormal cancer cells. Food Chem Toxicol 2008, 46, 1257-1270, doi:10.1016/j.fct.2007.09.082.
10. Flora, S.J. Role of free radicals and antioxidants in health and disease. Cell Mol Biol (Noisy-le-grand) 2007, 53, 1-2.
11. Singh, K., and Sharma, A. . Phytoconstituents and Therapeutic Potential of Allium cepa Linn. . PHCOG REV 2009, 5, 170-180.
12. Shenawy, N.S. Mitigating Effect of Ginger against Oxidative Stress Induced by Atrazine Herbicides in Mice Liver and Kidney. Journal of Biofertilizers & Biopesticides 2011, 02, doi:10.4172/2155-6202.1000107.
13. Galeone, C.; Pelucchi, C.; Levi, F.; Negri, E.; Franceschi, S.; Talamini, R.; Giacosa, A.; La Vecchia, C. Onion and garlic use and human cancer. Am J Clin Nutr 2006, 84, 1027-1032, doi:10.1093/ajcn/84.5.1027.
14. Kumar, K.P.S., Bhowmik, D., & Tiwari, P. . Allium cepa: A traditional medicinal herb and its health benefits. Journal of Chemical and Pharmaceutical Research 2010, 2, 283-291, doi: www.jocpr.com.
15. Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological Properties and Bioactive Components of Allium cepa L.: Focus on Potential Benefits in the Treatment of Obesity and Related Comorbidities. Molecules 2018, 24, doi:10.3390/molecules24010119.
16. A.B, M. Effects of Aqueous Extract of Onion (Allium cepa) on Blood Parameters in Adult Wistar Rats (Rattus novergicus). IOSR Journal of Pharmacy and Biological Sciences 2013, 5, 71-74, doi:10.9790/3008-0547174.
17. Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: their cancer prevention properties. Cancer Prev Res (Phila) 2015, 8, 181-189, doi:10.1158/1940-6207.CAPR-14-0172.
18. Upadhyay, R.K. Nutraceutical, pharmaceutical and therapeutic uses of Allium cepa: A Review. International Journal of Green Pharmacy 2016, 1, 46-64.
19. Roldan, E.; Sanchez-Moreno, C.; de Ancos, B.; Cano, M.P. Characterisation of onion (Allium cepa L.) by-products as food ingredients with antioxidant and antibrowning properties. Food Chem 2008, 108, 907-916, doi:10.1016/j.foodchem.2007.11.058.
20. Suru, S.M. Onion and garlic extracts lessen cadmium-induced nephrotoxicity in rats. Biometals 2008, 21, 623-633, doi:10.1007/s10534-008-9148-5.
21. Bhasker, P., Tailor, A. K., Sharma, H. P., Singh, R. K., and Gupta, P. K. . Medicinal, Nutraceutical Values and Consumption Pattern of Onion (Allium cepa) in India: An Over View. . International Journal of Current Microbiology and Applied Sciences 2018, 2629-2638.
22. Ozougwu, J.C. Anti-diabetic effects of Allium cepa (onions) aqueous extracts on alloxan-induced diabetic Rattus novergicus. Journal of Medicinal Plants Research 2011, 5, 1134-1139, doi:10.5897/jmpr.
23. Dhaslin, Y.F., Issac, R., and Prabha, M. L. . Antioxidant, antimicrobial, and health benefits of nutmeg. . Drug invention Today 2019, 12, 167-169.
24. Erukainure, O.L.; Ajiboye, J.A.; Abbah, U.A.; Asieba, G.O.; Mamuru, S.; Zaruwa, M.Z.; Manhas, N.; Singh, P.; Islam, M.S. Monodora myristica (African nutmeg) modulates redox homeostasis and alters functional chemistry in sickled erythrocytes. Hum Exp Toxicol 2018, 37, 458-467, doi:10.1177/0960327117712385.
25. Ola-Mudathir, K.F.; Suru, S.M.; Fafunso, M.A.; Obioha, U.E.; Faremi, T.Y. Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem Toxicol 2008, 46, 3604-3611, doi:10.1016/j.fct.2008.09.004.
26. A. Enabulele, S.; Oboh, F.O.J.; O. Uwadiae, E. Antimicrobial, Nutritional and Phytochemical Properties of Monodora Myristica Seeds. IOSR Journal of Pharmacy and Biological Sciences 2014, 9, 01-06, doi:10.9790/3008-09430106.
27. Kwino, D.I., Okonkwo, W. I., and Obi, O. F. . Effects of roasting on the Physical Properties of Monodora myristica (African nutmeg). International Food Research Journal 2017, 24, 1148-1155.
28. Moukette, B.M.; Pieme, C.A.; Njimou, J.R.; Biapa, C.P.; Marco, B.; Ngogang, J.Y. In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non-timber forest product used as spice: Monodora myristica. Biol Res 2015, 48, 15, doi:10.1186/s40659-015-0003-1.
29. Nwozo Sarah Onyenibe , K.T.F., Oyinloye Babatunji Emmanuel African Nutmeg (Monodora Myristica) Lowers Cholesterol and Modulates Lipid Peroxidation in Experimentally Induced Hypercholesterolemic Male Wistar Rats. International journal of Biomedical science 2015, 11, 86-92.
30. Maduike, E.; Anuna, N. Infrared Spectroscopy and Microorganisms Associated with African Nutmeg (Monodora myristica) Seeds Sold in a Municipal Market in Imo State, Nigeria. Asian Journal of Research in Medical and Pharmaceutical Sciences 2018, 3, 1-7, doi:10.9734/ajrimps/2018/40661.
31. Olatoye, M.O.; Marla, S.R.; Hu, Z.; Bouchet, S.; Perumal, R.; Morris, G.P. Dissecting Adaptive Traits with Nested Association Mapping: Genetic Architecture of Inflorescence Morphology in Sorghum. G3 (Bethesda) 2020, 10, 1785-1796, doi:10.1534/g3.119.400658.
32. Ishiwu, C.N., Udedi, S. C., and Ogeneh, B. O. . Original Research Article Evaluation of antioxidant potential of Monodora myristica (African Nutmeg). International Journal of Current Microbiology Applied of Sciences 2013, 2, 373-383, doi:
33. Agiriga, A.; Siwela, M. Monodora myristica (Gaertn.) Dunal: A Plant with Multiple Food, Health and Medicinal Applications: A Review. American Journal of Food Technology 2017, 12, 271-284, doi:10.3923/ajft.2017.271.284.
34. Edak, A.U.; Chukwudi, U.; Peter, O.A. Genetic diversity in African nutmeg (Monodora myristica) accessions from South Eastern Nigeria. African Journal of Biotechnology 2014, 13, 4105-4111, doi:10.5897/ajb2014.14075.
35. Alam, K., Hoq, O., and Uddin, S. Medicinal plant Allium sativum = A Review. Journal of Medicinal Plants Studies 2016, 4, 72-79.
36. Phan, A.D.T.; Netzel, G.; Chhim, P.; Netzel, M.E.; Sultanbawa, Y. Phytochemical Characteristics and Antimicrobial Activity of Australian Grown Garlic (Allium Sativum L.) Cultivars. Foods 2019, 8, doi:10.3390/foods8090358.
37. Stavělíková, H. Morphological characteristics of garlic (Allium sativum L.) genetic resources collection. Information 2008, DOI : 10.17221 / 661-HORTSCI, 130-135, doi:DOI : 10.17221 / 661-HORTSCI.
38. Huzaifa, U., Labaran, I., Bello,A.B. and Olatunde, A. . Phytochemical Screening of Aqueous Extract of Garlic (Alliumsativum) bulbs. Report and Opinion 2014, 7, 5371-5376, doi:doi:10.7537/j.issn.1553-9873.
39. Ali, M., and Ibrahim, I. S. Phytochemical Screening and Proximate Analysis of Garlic (Allium Sativum). Archive of organic and inorganic chemical sciences 2019, 478-482,
40. Musavi, H.; Tabnak, M.; Alaei Sheini, F.; Hasanzadeh Bezvan, M.; Amidi, F.; Abbasi, M. Effect of garlic (Allium sativum) on male fertility: a systematic review. Journal of Herbmed Pharmacology 2018, 7, 306-312, doi:10.15171/jhp.2018.46.
41. Evans, W.C., Evans, D., and Trease, G. E. 2002. . Trease and Evans’ pharmacognosy. WB Saunders. 2002.
42. Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. The Japanese Journal of Nutrition and Dietetics 1986, 44, 307-315, doi:10.5264/eiyogakuzashi.44.307.
43. Greenwald, R.A. CRC handbook of methods for oxygen radical research. CRC Press 1985, https://doi.org/10.1201/9781351072922
44. Wolff, S.P. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods in Enzymology 1994, 233, 182-189, doi:https://doi.org/10.1016/S0076-6879(94)33021-2.
45. Varshney, R.; Kale, R.K. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol 1990, 58, 733-743, doi:10.1080/09553009014552121.
46. He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol Biochem 2017, 44, 532-553, doi:10.1159/000485089.
47. Tan, A.C.; Konczak, I.; Sze, D.M.; Ramzan, I. Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr Cancer 2011, 63, 495-505, doi:10.1080/01635581.2011.538953.
48. Harris, R.Z.; Jang, G.R.; Tsunoda, S. Dietary effects on drug metabolism and transport. Clin Pharmacokinet 2003, 42, 1071-1088, doi:10.2165/00003088-200342130-00001.
49. Akinmoladun, A.C., Ibukun, E. O., Afor, E., Obuotor, E. M., and Farombi, E. O. Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimum. Scientific Research and Essays 2007, 2, 4, doi:10.5897/sre.
50. Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003, 3, 768-780, doi:10.1038/nrc1189.
51. Owumi, S.E.; Oloidi, A.C.; Oloye, C.O.; Oladeji, O.O.; Obadare, M.O.; Odunola, O.A. Toxicological and phytoprotective effect of Keayodendron bridelioides and Monodora myristica extracts in Wister rats. Pharmacognosy Res 2015, 7, S26-33, doi:10.4103/0974-8490.150508.
52. Agbor, G.A.; Moumbegna, P.; Oluwasola, E.O.; Nwosu, L.U.; Njoku, R.C.; Kanu, S.; Emekabasi, E.I.; Akin, F.; Obasi, A.P.; Abudei, F.A. Antioxidant capacity of some plant foods and beverages consumed in the Eastern Region of Nigeria. Afr J Tradit Complement Altern Med 2011, 8, 362-369, doi:10.4314/ajtcam.v8i4.4.
53. Kim, J.S., and Kim, M. J. 2010. . In vitro antioxidant activity of Lespedeza cuneata methanolic extracts. Journal of Medicinal Plants Research 2010, 4, 674-679, doi: DOI: 10.5897/JMPR09.
54. Geethangili, M.; Ding, S.T. A Review of the Phytochemistry and Pharmacology of Phyllanthus urinaria L. Front Pharmacol 2018, 9, 1109, doi:10.3389/fphar.2018.01109.
55. Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 2011, 21, 103-115, doi:10.1038/cr.2010.178.
56. Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43-50, doi:10.1016/s0300-483x(00)00231-6.
57. Alche, J.D. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol 2019, 23, 101136, doi:10.1016/j.redox.2019.101136.
58. Dhingra, N., Sharma, R., and Kar, A. . Oxidative stress and carcinogenesis: Prevention by antioxidative phytochemicals acting on different molecular targets. . International Journal of Pharmaceutical Sciences Review and Research 2014, 25, 236-245.
59. Kang, O.J. Physicochemical Characteristics of Black Garlic after Different Thermal Processing Steps. Prev Nutr Food Sci 2016, 21, 348-354, doi:10.3746/pnf.2016.21.4.348.
60. Park, C.; Park, S.; Chung, Y.H.; Kim, G.Y.; Choi, Y.W.; Kim, B.W.; Choi, Y.H. Induction of apoptosis by a hexane extract of aged black garlic in the human leukemic U937 cells. Nutr Res Pract 2014, 8, 132-137, doi:10.4162/nrp.2014.8.2.132.
61. Tamfu, A.N.; Ceylan, O.; Kucukaydin, S.; Ozturk, M.; Duru, M.E.; Dinica, R.M. Antibiofilm and Enzyme Inhibitory Potentials of Two Annonaceous Food Spices, African Pepper (Xylopia aethiopica) and African Nutmeg (Monodora myristica). Foods 2020, 9, doi:10.3390/foods9121768.
62. Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, doi:10.3390/foods8070246.
63. Scaglione, C.N.; Xu, Q.; Ramanujan, V.K. Direct measurement of catalase activity in living cells and tissue biopsies. Biochem Biophys Res Commun 2016, 470, 192-196, doi:10.1016/j.bbrc.2016.01.026.
64. Bongiorno, P.B.; Fratellone, P.M.; LoGiudice, P. Potential Health Benefits of Garlic (Allium Sativum): A Narrative Review. Journal of Complementary and Integrative Medicine 2008, 5, doi:10.2202/1553-3840.1084.












