1.0 Introduction.
Urinary tract infections (UTI) are serious health problems affecting millions of people each year. They are the second most common type of infection in the body, accounting for about 8.3 million visits to the hospitals each year [1]. UTIs are caused by the presence of bacteria in urine, although fungi and viruses could be involved. The majority of women have recurrent infections within one year [2]. Escherichia coli causes 75 – 90% of uncomplicated UTIs [3] whereas Staphylococcus saprophyticus causes an estimated 5 – 15% of UTIs frequently in younger women [4].
Enterococcus and other Gram-Negative rods other than E. coli have also been implicated in some cases [5]. Significant bacteria can be defined as the isolation of persistent bacteria Colony Forming Units (CFU) per mL of clean voided, mid-stream urine specimens plated within 6 hours of collection. In females, it is possible that slow-growing Microaerophiles such as Lactobacillus, Corynebacterium and Streptococcus mileri may be involved in the pathogenesis of the infections. Symptoms are usually precipitated by sexual intercourse [4]. UTIs occur in both acute and chronic forms. In the former, patients complain of severe and low back pain that may associate with fever due to the associated bacteraemia, while in the latter, a sensation of perennial fullness is felt [6].
The common causative agent is E. coli but micrococcal infections may account for up to 10 – 20% of cases in sexually active women [6]. This infection reaches the bladder by the ascending route, with the main symptoms as urinary frequency and dysuria [3]. Other infections that are due to less common pathogens usually occur in the presence of gross structural abnormality of the urinary tract or neurological effects [1]. The common source of E. coli infections in women is the faecal flora. Introital colonization precedes the development of urinary tract infections in women and girls. In males, the organisms frequently originate from the sub-pepucial sac [7].
The higher prevalence in females as compared with males is attributable to the shortness of the female urethra and so is more liable to contamination during sexual intercourse, urethra massage and even urination with chronic flora that resides in the perineal skin. It also includes the effect of turbulence on the urinary Stream [7].
The present study evaluated the presence or absence of bacteria in the urine samples, isolated and identified the most prevalent bacteria associated with UTIs and identified the drug sensitivity patterns of the isolates from student residents of Nicon hostel of the Federal Polytechnic Bida.
2.0 Materials and Methods
2.1 Studied Population
Female students of Nicon Hostel resident of the Federal Polytechnic Bida were recruited for this study. All recruited students gave their consent for participation in the study and Ethical approval was also sought from The Federal Polytechnic Bida Medical Centre.
2.2 Ethics Approval and Consent:
Ethical clearance was obtained from the Research and Ethical Review Committee and was approved by the Department of Biological Sciences, School of Applied and Natural Sciences, Federal Polytechnic Bida. Ethical clearance was also secured from Federal Polytechnic Bida Medical Center. Official permission was also obtained from the HOD of the department and the Director of The FPB Medical centre. In addition, written informed consent was obtained from the study participants before the initiation of data collection and all participants were informed about the purpose of the study. The individual results of the investigations remained confidential. The laboratory findings of the study participants were communicated to the responsible health professional assigned to the laboratory for the purpose of managing the cases accordingly.
2.3 Determination of sample size
The sample size was determined by using the equations below
N = Z2 x P x (1- P)
D2
N = (1.96)2 x 0.146 x (1- 0.146)
(0.05)2
N = 3.8 x 0.146 x 0.854
0.0025
N = 0.4737992
0.0025
N = 189.51968, N = 190
Where
N = sample size (190), D = margin of error (0.05), Z = statistics for a level of confidence 95%, P = prevalence of UTI 14.6% (based on retrospective study results of Jombo et al. [8].
2.4 Sample collection
Clean-voided, mid-stream urine (MSU) specimens were collected from the female students residing in Nicon hostel the federal polytechnic Bida. Each of the students was instructed on the mode of collection of the MSU that is, during forceful urination after the first 10 – 20 ml has been voided. The subjects were educated adequately on the precautions to prevent contamination of specimens. The specimens were collected in sterilized, wide-necked, leak-proof, plastic (specimen bottles) [9].
2.5 Dipstick Urinalysis (combo10 stick)
The test strip was dipped approximately 1 second into the fresh urine. The strip was drawn across the rim of the container to remove excess urine. After 30 to 60 seconds (leukocyte test field after 60 – 120 seconds), the test strip was compared with the colour scale. The best time for comparison is after 30 seconds because colour changes that take place after more than 2 minutes are of no significant [10].
2.6 Isolation of Uropathogens
Primary isolation was done on CLED Agar and Nutrient Agar to allow the growth of all bacteria in the urine. Each specimen of urine was shaken properly to ensure homogeneity. Using a sterilised wire loop sterilized at red hot on a Bunsen flame and allowed to cool, a little of the urine sample was picked using the sterile wire loop. This was used as inoculums using the streak plate method [11]. Growth identified on plates is indicated as significant bacteria.
2.7 Identification of Uropathogens
All bacterial isolates were characterized on the basis of colonial morphology, reaction to Gram’s stain, motility test, [12] and coagulase tests. The tentative identities of the isolate were obtained from Bergey’s Manual of Determinative Bacteriology [13].
2.8 Sensitivity tests
Nutrient Agar was used for the antibiotics sensitivity test. The method described by Singh et al. [14] was employed. The bacterial isolates were subjected to in vitro susceptibility tests against some common third-generation antibiotics.
3.0 Results
3.1 Percentage frequency of occurrence of the characterized isolates
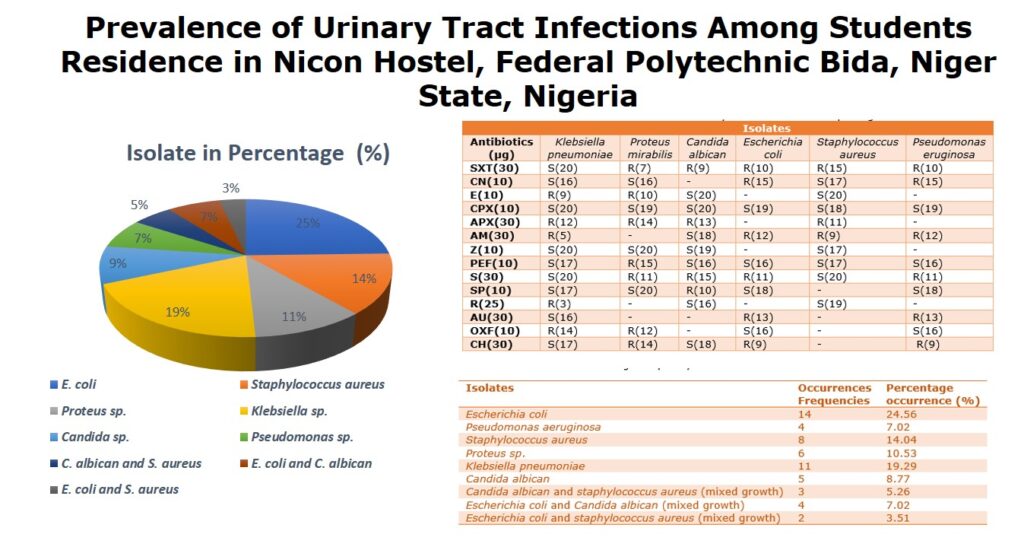
A total of one hundred and ninety urine samples were analyzed. Fifty-seven (30%) of the samples were positive for uropathogens while one hundred and thirty-three (70%) samples were negative for uropathogens. The fifty-seven (57) positive samples were either Nitrite (29) or leukocyte (28) positive. The isolates were tentatively identified to belong to six (6) genera including Escherichia coli, Klebsiella sp., Staphylococcus sp., Proteus sp., Candida sp. and Pseudomonas sp. E. coli shows the highest prevalence (24.56%) and Pseudomonas sp. (7.02%) has the least prevalence. The Cultural characterization of the isolates is presented inTable 1. Out of the fifty-seven (57) significant bacteria isolate, the frequency of occurrence of the uropathogens includes; 14 Escherichia coli, 4 Pseudomonas aeruginosa, 8 staphylococcus aureus, 6 Proteus mirabilis, 11 Klebsiella pneumoniae, 5 Candida albican withpercentagefrequencyofoccurrenceas24.56%, 7.02%, 14.04% 10.53%, 19.29% and 8.77% respectively. Nine (9) isolate of the significant bacteria isolated were mixed growth which include; 3 (5.26%) Candida albican and Staphylococcus aureus, 4 (7.02%) Escherichia coli and Candida albican and 2 (3.51%) Escherichia coli and Staphylococcus aureus (Table 2, Figure 1).
Table 1: Cultural characterization of the isolates
| Isolates | CLED Agar | Nutrient Agar | Gram Stain | Motility Test | Coagulase Test | Suspected Organism |
| A | Opaque yellow colonies with a slightly deeper yellow center | Large, thick, grayish white, moist, smooth, opaque or translucent discs with some mucoid strain | Gram negative bacilli, exist singly or in pairs | Motile | Negative | Escherichia coli |
| B | Green colonies with typical matte surface and rough periphery | Greenish colouration, large, opaque, flat colonies with irregular margins and distinctively fruity odour colonies | Gram-negative, rod shaped, asporogenous, monoflagellate, has apearlescent appearance | Motile | Negative | Pseudomon as aeruginos |
| C | Deep yellow colonies about 0.75mm in diameter, uniform in colour on yellow medium | Round, smooth, convex, glistening with entire edge golden yellow coloured colonies | Gram-positive and appear in spherical shape. They are often in clusters resembling bunch of grape | Non- motile | Positive | Staphylococ cus aureus |
| D | No swarming, no visible growth within 24hrs | Smooth, pale or colourless colonies with successive waves that form a thin filmy layer which colonizes the whole plate within 24hr | Gram-negative, non-capsulated, rod-shaped, hyper-flagellated and elongated with swarmer cells | Motile | Negative | Proteus mirabilis |
| E | Large, mucoid colonies | Dome-shaped, Mucoid, Translucent–Opaque which is Greyish white | Gram-negative encapsulated rod- shaped, appears singly and in pairs or short chain | Non- motile | Negative | Klebsiella pneumonia |
| F | Tiny to small whitish colonies on a blue medium | Pasty and yellow- white filamentous colonies from which feet extend out from the marging into the surrounding agar | Gram-positive, encapsulated and diploid with filamentous cells which appears singly | Motile | Negative | Candida albican |
The tentative identities of the isolates were obtained from Bergey’s Manual of Determinative Bacteriology Buchanan and Gibson, [13].
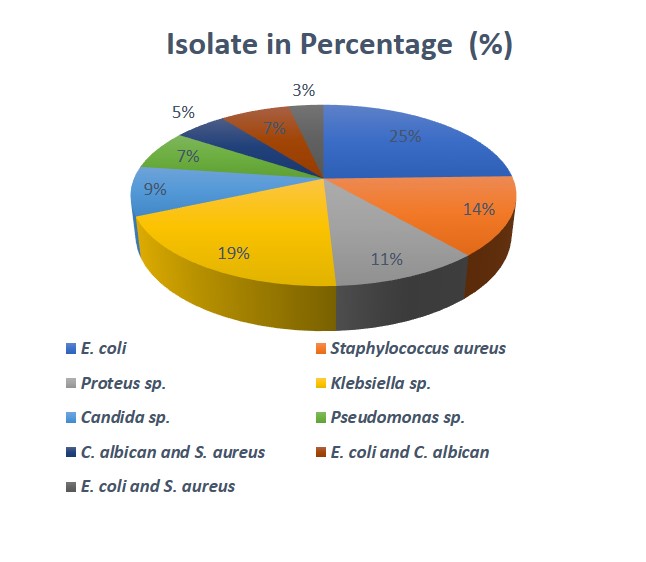
Table 2: Percentage frequency of occurrence of the characterized isolates
| Isolates | Occurrences Frequencies | Percentage occurrence (%) |
| Escherichia coli | 14 | 24.56 |
| Pseudomonas aeruginosa | 4 | 7.02 |
| Staphylococcus aureus | 8 | 14.04 |
| Proteus sp. | 6 | 10.53 |
| Klebsiella pneumoniae | 11 | 19.29 |
| Candida albican | 5 | 8.77 |
| Candida albican and staphylococcus aureus (mixed growth) | 3 | 5.26 |
| Escherichia coli and Candida albican (mixed growth) | 4 | 7.02 |
| Escherichia coli and staphylococcus aureus (mixed growth) | 2 | 3.51 |
3.2 Antibiotics sensitivity patterns of the isolated pathogens
In Table 3, One hundred and ninety (190) samples were examined for significant bacterial indicators of urinary tract infections. The analysis reveals that 57 (30%) isolates were positive for bacteria while 133 (70%) were negative. The fifty-seven (57) bacterial isolates were tentatively characterized into six genera. The frequencies of occurrence of the bacterial species are in the order: Escherichia coli (35.09%), Proteus sp. (10.53%), Klebsiella sp. (19.29%), Staphylococcus sp. (22.81%), Candida sp. (21.05%) and Pseudomonas sp. (7.02%) whereas mixed culture (15.79%). 57 (100%) were sensitive to ciprofloxacin, 51 (89.47%) to Pefloxacin, 41 (71.93%) to Sparfloxacin. Zinnacef, Gentamycin and streptomycin had a relatively fair activity on the isolates with 37 (64.91%), 30 (52.63%) and 24 (42.1%) respectively. However, bacterial isolates obtained from this survey generally showed a similar pattern of resistance to antibiotics. A particularly remarkable result was obtained with Ciprofloxacin and Pefloxacin.
It was seen that out of the 57 isolates, 100% were sensitive to ciprofloxacin and 89.47% were sensitive to Pefloxacin. Earlier research also revealed that the success of ciprofloxacin could be due to its broad-spectrum activities, its bactericidal activity on organisms both in replicating and resting state and its ability to disrupt DNA functions leading to the death of the bacterium. Sparfloxacin was also similar in action to ciprofloxacin, recording 71.93% of sensitive cases. In that, it is equally highly recommended. Drugs like Zinnacef, Gentamycin and streptomycin have a relatively fair activity on isolates with 64.91%, 52.63%and 42.1% activities respectively. The recorded high level of resistance could be due to the quantity and concentration of some chemicals used in the production of these antibiotics and the fact that these antibiotics are also produced to handle other viral diseases. This may lead to the development of resistance. On the other hand, Ampiclox recorded a very poor performance with 21.05% sensitivity.
In general, antibiotics like ciprofloxacin (CPX) at 10µg and pefloxacin (PEF) at 10µg showed excellent sensitive inhibitory actions while the activity of Ampiclox (APX) at 30µg is not commendable because it inactivity in UTI cases is attributable to the fact that it was manufactured for activity against anaerobic microorganisms and not aerobes as the case is for the isolates.
Table 3: Antibiotics sensitivity test on the isolated pathogens
| Isolates | ||||||
| Antibiotics (µg) | Klebsiella pneumoniae | Proteus mirabilis | Candida albican | Escherichia coli | Staphylococcus aureus | Pseudomonas eruginosa |
| SXT(30) | S(20) | R(7) | R(9) | R(10) | R(15) | R(10) |
| CN(10) | S(16) | S(16) | – | R(15) | S(17) | R(15) |
| E(10) | R(9) | R(10) | S(20) | – | S(20) | – |
| CPX(10) | S(20) | S(19) | S(20) | S(19) | S(18) | S(19) |
| APX(30) | R(12) | R(14) | R(13) | – | R(11) | – |
| AM(30) | R(5) | – | S(18) | R(12) | R(9) | R(12) |
| Z(10) | S(20) | S(20) | S(19) | – | S(17) | – |
| PEF(10) | S(17) | R(15) | S(16) | S(16) | S(17) | S(16) |
| S(30) | S(20) | R(11) | R(15) | R(11) | S(20) | R(11) |
| SP(10) | S(17) | S(20) | R(10) | S(18) | – | S(18) |
| R(25) | R(3) | – | S(16) | – | S(19) | – |
| AU(30) | S(16) | – | – | R(13) | – | R(13) |
| OXF(10) | R(14) | R(12) | – | S(16) | – | S(16) |
| CH(30) | S(17) | R(14) | S(18) | R(9) | – | R(9) |
Keys: R = Resistance (0-15), S =Sensitivity (16 -20), – = (<0). SXT =Septrin, CN =Gentamycin, E =Erythromycin, CPX =Ciprofloxacin, APX =Ampiclox, AM =Amoxacillin, Z =Zinnacef, PEF =Pefloxacin, S = streptomycin, SP =sparfloxacin, R =Rocephin, AU = Augmentin, OFX =Tarivid, CH =chloramphenicol.
4.0 Discussion
The dipstick urinalysis (combo 10 stick), nitrate reduction test is based on the reduction of nitrate in the urine (from diet) to nitrite by the action of gram-negative bacteria. The test is most accurate on a first-morning urine specimen or on a sample that has been collected 4h or more after the last voiding to allow time for organisms in the bladder to metabolize the nitrate. The test is reasonably effective in identifying infection due to members of the family Enterobacteriaceae but fails to identify infection due to gram-positive organisms, Pseudomonas spp., or yeast [10].
Pyuria is demonstrated in almost all acute bacterial UTI, and its absence should call the diagnosis into question. The leukocyte esterase test for pyuria will detect both lysed and intact leukocytes and is based on the presence of intracellular esterases that catalyze the hydrolysis of esters, releasing components that yield a purple colour. The leukocyte esterase test is less sensitive than microscopy in identifying pyuria but is a useful alternative when microscopy is not feasible [15].
Ascorbic acid concentrations of 25 mg/dl or greater may cause false-negative results in specimens containing only small amounts of nitrite. Other interfering substances include elevated glucose concentrations, high specific gravity, the presence of cephalexin or cephalothin, and high concentrations of oxalic acid or tetracycline [15].
One hundred and ninety (190) urine specimens were examined for presence of bacteria or otherwise. Fifty-seven (30%) were found positive for urinary tract infections. However, 133 (70%) other specimens are found negative. The study implicated six microorganisms as possible Aetiological agents of the UTI cases observed. According to Mars [16], the common causative agents of UTI are E. coli, Proteus sp., Klebsiella sp., Staphylococcus sp., Candida sp. and Pseudomonas sp. which are in agreement with the organisms identified in this study. This higher prevalence of E. coli (24.56%) may be due to faecal contamination, the predilection of the organisms from the toilets and the shortness of the female urethra [17].
This prevalence however, is also reported in earlier works by Smith et al. [18], where they found out that E. coli accounts for 32% of UTI cases, Proteus sp. with 17.25% prevalence has a significant association with UTI. Its active motility and swarming ability can in comparison with other organism’s transverse easily through the urethra. Other organisms implicated were Klebsiella sp. (13.79%), Staphylococcus sp. (12.07%), Streptococcus sp. (8.63%) and Pseudomonas sp. (5.17%).
Six of the positive cases were caused by a mixed culture of these organisms accounting for 10.34%. In this study, Proteus sp. with 10.53%, Klebsiella pneumoniae (19.29%), Staphylococcus aureus (14.04%), Candida albican (8.77%), and Pseudomonas sp. (7.02%) which are in agreement with the organisms identified with the previous work. Nine of the positive cases were caused by a mixed growth of these organisms accounting for 15.79%, which are; Candida albican and staphylococcus aureus (5.26%), Escherichia coli and Candida albican (7.02%) and Escherichia coli and staphylococcus aureus (3.51%).
There is also a possible link between the prevalence of UTI among students and the level of personal hygiene or the state of toilet facilities in the hostels. From the private oral interview conducted with some of these students, most of the students rated the hostel toilets as bad. Bad, in this context implies that there is no adequate supply of water to clean and flush the toilets regularly when dirty. Therefore, there is an accumulation of urine sediments forming a thick scum. In this case students could become infected during urination. In this study, it was observed that the susceptibility of the isolates to the fourteen antibiotics tested differs with the species due to their genetic makeup and their cellular morphology. A particularly remarkable result was obtained with Ciprofloxacin and Pefloxacin.
It was seen that out of the 57 isolates, 100% were sensitive to ciprofloxacin and 89.47% sensitive to Pefloxacin. Earlier researches also revealed that the success of ciprofloxacin could be due to its broad spectrum activities, its bactericidal activity on organisms both in replicating and resting state and its ability to disrupt DNA functions leading to the death of the bacterium. Sparfloxacin was also similar in action as ciprofloxacin, recording 71.93% sensitive cases. In that, it is equally highly recommended.
Drugs like Zinnacef, Gentamycin and streptomycin have a relatively fair activity on isolates with 64.91%, 52.63%and 42.1% activities respectively. The recorded high level of resistance could be due to the quantity and concentration of some chemicals used in the production of these antibiotics and the fact that these antibiotics are also produced to handle other viral diseases. This may lead to the development of resistance.
On the other hand, Ampiclox recorded a very poor performance with 21.05% sensitivity. In general, antibiotics like ciprofloxacin (CPX) at 10µg and pefloxacin (PEF) at 10µg showed excellent sensitive inhibitory actions while activity of Ampiclox (APX) at 30µg is not commendable because it’s inactivity in UTI cases is attributable to the fact that it was manufactured for activity against anaerobic microorganisms and not aerobes as the case is for the isolates. Those that are negative were only 133 (70%). As earlier reported by several researchers, E. coli was also implicated as the most common causative agent of UTI among female students of this study.
5.0 Conclusion
Prevalence of UTI is high among the students of Federal Polytechnic Bida student residing in Nicon hostel when compared to other studies. This is a matter of concern. Lack of awareness regarding these risk factors, lack of time to spend for one’s health, busy schedules may be some factors which could play in this aspect.
Author Contributions: Suleiman, A.: Design the research plan, organized the study and contributed to the writing of the manuscript.: Adole, A.T.: Researched, collected the data, conducted the practical, reviewed the table and wrote the manuscript.
Funding: This study was sponsored by the Authors.
Conflict of Interest: The authors declare that they have no conflicts of interest for this work.
Data Availability Statement: Data is available upon request.
Consent for Publication: Not applicable.
Acknowledgments: The authors would like to thank The Federal Polytechnic Bida Medical Center and Department of Biological Sciences for sponsoring the research. Our special thanks and appreciation goes to the medical director and laboratory staff working in the school medical for their support in screening of symptomatic and asymptomatic study participants and organizing the preconditions of sample and data collection. Our deepest thanks also goes to the Federal Polytechnic Bida Directorate for their unreserved material and reagent supply that made this study possible. We also extend our profound gratitude to the study participants for their willingness to engage, without whom this research would not have been possible.
References
1. UDHHS (2004). Vital and Health Statistics 13(1): 157.
2. Siiri, K., Ka,T., Inga, V., Jelena, S., Epp, S. andMarika, M. (2009). Persistence of Escherichia coli clones and phenotypic and genotypic antibiotic Resistance in recurrent urinary tract infection in childhood. Journal of Clinical Microbiology 47: 99-105.
3. Karen, H., Dorthe, S., Bettina, L., Suen, F., Stig, L., Tor, M., Rolf, L. and Niels, F. (2006). Pulse-field gel electrophoresis typing of Escherichia coli strains from samples collected before and after pivmeecillinam or Placebo treatment of uncomplicated community acquired urinary tract Infection in women. Journal of Clinical Microbiology 44: 1776-1781.
4. Micheal, W., Johan, W., Suen, F., Carina, K. and Tor, M. (2007). Molecular Epidemiology of Staphylococcus saprophyticus isolated from women With uncomplicated community- acquired urinary tract infection. Journal of Clinical Microbiology 45: 1561-1564.
5. Benjamin, W. D., Brian K. P. and Gary V. D. (2009). Lactobacillus delbrueckii as the cause of urinary tract infection. Journal of Clinical Microbiology 47: 275-277.
6. Blango, M. G., Ott, E. M., Erman, A., Veranic, P. & Mulvey, M. A. Vorland, L.H., Carlson, K. and Aaeelen, O. (2001). Antibiotics resistance and Small v-plasmid among E.coli isolates from out patient UTIs in Northern
7. Norway. Antimicrobial. Agents and Chemotherapy 27(1): 107-113.
Starr, C. and Taggart, R.C. (2002). Biology: the unit and diversity of life. Wadsworth publishing company Belmont California pp. 509-533.
8. Jombo, G. T., Egah, D. Z., Banwat, E. B. and Ayeni, J. A. (2006). “Nosocomial and community acquired urinary tract infections at a teaching hospital in north central Nigeria: findings from a study of 12,458 urine samples,” Nigerian Journal of Medicine, vol. 15, no. 1, pp. 230–236
9. Laurence Knott, and Helen Huins. (2018). Midstream Specimen of Urine (MSU).
10. Wilson, M. L., and Gaido, L. (2004). Laboratory diagnosis of urinary tract infections in adult patients. Clinic of Infectious Diseases 38:1150–1158.
11. Nonzon, O. T., Ory, E. M. and Debson, M. L. (2002). A comparison of bacterial Counts in the urine obtained by needle aspiration of the bladder, Catheterization and mid stream voided method. New England Journal of Medicine 259: 64.
12. Fawole, E.O. and Oso, O.O. (2007). An introduction to laboratory Manual of Microbiology. University printing press Ibadan, Nigeria pp. 23-34.
13. Buchanan, R. E. and Gibson, N. E. (1974). Bergey’s Manual of Determinative Bacteriology
14. Singh, M., Subramanian, M., Ganesapandian, S. and Kumaraguru, A. (2011). Antimicrobial susceptibility pattern of urinary tract infection causing human pathogenic bacteria. Asian Journal of Medical Sciences 3 (2), 56-60.
15. Hooton, T. M., and Stamm, W. E. (1977). Diagnosis and treatment of uncomplicated urinary tract infection. Infectious Disease Clinic Of North America 11:551–581.
16. Mars, P. S. (2002). Urinary tract infection. Merck manual of diagnosis and Therapy. Merck research laboratory. Railway New Jersey pp. 11784-11798.
17. Nicolle, L. E. (2001). Epidemiology of urinary tract infection. 11: 551-564.
18. Smith, P. B., Barry, A .L. and Truck. M. (2003). Laboratory Diagnosis of Urinary Tract Infections. American Society for Microbiology 2: 1-7