1.0 Introduction.
The global market for industrial enzymes is increasing from year to year, it constitutes the largest product segment in the global industrial enzymes sales in various industrial market sectors such as detergent, food, pharmaceutical, leather, diagnostics, waste management and silver recovery [1]. Considering this market demand, there is a need to investigate new microorganisms because they are the major sources of all commercially important alkaline proteases, which have unlimited industrial applications [2]
Proteases are produced from different groups of organisms such as bacteria, yeast, fungi, plants and animals [3]. Bacteria, mainly the Bacillus group, are the major source of commercial proteases since they easily produce extracellular enzymes using fermentation techniques, thus enabling simple downstream processing[3]. The characteristics of proteases from Bacillus vary, depending on the conditions of their native habitat [4]. For example, proteases produced by a mesophilic Bacillus subtilis isolated from soil sample has optimum activity at 40°C and neutral pH [5]. Meanwhile, a thermophilic Bacillus toebii strain LBT 77 isolated from a hot spring has optimum activity at 95°C and pH 13. The activity of the enzyme increases with the addition of 25% acetonitrile, methanol, ethanol, and n-butanol [6].
Agricultural-based industries produced a vast number of residues every year [7]. If these residues are released to the environment without proper disposal procedure that may cause environmental pollution and harmful effect on human and animal health [8]. Most of the agro-industrial wastes are untreated, underutilized, and are therefore disposed either by burning, dumping or unplanned landfilling [9]. These untreated wastes create different problems with climate change by increasing the number of greenhouse gases. Besides this, the use of fossil fuels also contributes to the effect of greenhouse gases [10]. It is therefore important to seek for alternative cleaner and renewable bioenergy resources [11].
These wastes cause a serious disposal problem. For example, the juice industries produced a huge amount of waste as peels, the coffee industry produced coffee pulp as waste, and cereal industries produced husks. All over the world approximately 147.2 million metric tons of fibre sources are found, whereas 709.2 and 673.3 million metric tons of wheat straw residues and rice straws were estimated, respectively, in the 1990s [12]. As per the composition of these agro-industrial residues are concerned, they have a high nutritional perspective, therefore they are getting more consideration for quality control and are also categorized as agro-industrial by-products [13].
Groundnut shells account for approximately 20% of the dried peanut pod by weight, meaning there is a significant amount of shell residual left after groundnut processing[14]. Increased groundnut production leads to the accumulation of these groundnut shells which is not utilized, thus either burnt or buried. As Groundnut shells are rich in many functional compounds, it can be utilized in multiple ways [15].
Beans are extensively grown in different parts of the world and, in particular, in the Mediterranean region [16]. Bean can be used as a dietary item alone or can serve as a potential supplement to cereal diets, especially for the preparation of inexpensive protein-rich food for children [17]. It contains 25.2% of proteins, 46.5% of carbohydrates, 1.5% of lipids and 10.3% of dietary fibre[18]. Beans are usually available throughout the year as fresh, frozen and fully mature. Due to the high consumption of beans, massive amounts of the peels (as waste) are disposed of, causing a severe problem in the community [19].
In the interest of the environment, we utilized this agricultural waste as a carbon source for microbial protease production. Therefore, the aim of this study is to isolate, partially characterize and produce protease from microorganisms isolated from the cow rumen ingesta using groundnut shell and Bean chaff as the carbon sources
2.0 Materials and methods
2.1 Media, chemical and reagent
Nutrient agar (NA), glucose, 0.87% sodium saline, 1% CaCO3, 70% and 95% alcohol, crystal violet (0.1g), gram’s iodine (0.18g), saffranine (0.2g), glycerine, 3% KOH, Malachite green (0.5g), carbolfuchsin stain, hydrochloride acid (conc. 3ml), methylene blue chloride (0.3g) beef extract (0.3%), zinc chloride (1g), potassium iodine (0.1g), powdered zinc metal, yeast extract (0.5g), MgSO4 (0.02g), K2HPO4 (0.1g), NaCl (0.5g), methyl red (0.008g), tryptone (1g), potassium phosphate (0.5%), sodium citrate (0.2g), agar (1.5g), bromomethyl blue (0.08g), (NH4)H2PO4 (0.1g).
2.2 Collection of samples
The dump site was collected in a sterile polythene bag from the sewage sludge wastes site and immediately transferred to the laboratory for the isolation of microorganisms. The groundnut shell and Bean chaff were obtained from Kpakungo Local Government area of Minna, Nigeria
2.3 Proximate analysis of groundnut shell and Bean chaff
The proximate compositions including; crude proteins, crude fibre, moisture content, ash content, crude fat and carbohydrate contents of both groundnut shell and Bean chaff were determined using standard procedures described in previous studies [20-22]
2.4 Isolation of microorganism
One gram (1gm) of domestic dumpsite sample was mixed with 9ml of saline solution (Master dilution) and 1ml of the solution was serially transferred to tubes containing 9 ml saline each so that for each transfer the suspension was diluted 10 times [23,24]. Each tube was shaken vigorously. 0.1ml solution was spread to Petri plates containing sterilized nutrient agar and saboroud dextrose agar for bacterial and fungal isolation. The pure isolates were stored in bottles for further studies [25,26].
2.5 Identification of Proteolytic Bacteria Isolated from Soil
The selected potential strain was then identified by morphological and biochemical characteristics by using the microbiology laboratory manual [27]. In addition, the morphological properties including the cell shape, pigmentation, fluorescence, spore shape and hyphae (fungi) of the isolated bacteria were studied using a microscope.
2.6 Screening for proteolytic activity
The isolates obtained from domestic dumpsite were spread on Petri plates containing milk agar medium (pH7) and incubated for 24h at 37°C and 5 days at 25oC for bacterial and fungal isolates respectively. A clear zone of skim milk hydrolysis indicated protease producing organisms. Colonies showing proteolytic activity were selected for protease enzyme production [28].
2.7 Production of Protease Enzyme by Submerged Fermentation
Protease production was carried out by inoculating protease producing isolate into a basal medium (NH4Cl-0.5%, NaCl-0.5%, CaCl2-0.2%, MgCl2.6H2O-0.2%, K2HPO4-0.4%, KH2PO4 0.3%) containing 0.7% peptone and 0.5 % as nitrogen and carbon source respectively. The mixture was adjusted to pH 7.5 and maintained at 37oC on a shaker at 250 rev/min for 96 hours. Samples were withdrawn and centrifuged every 12 hours and the supernatant was regarded as a crude protease enzyme [29].
2.8 Determination of Protease Enzyme Activity
The activity of protease was assessed in triplicate by measuring the release of trichloroacetic-acid soluble peptides from 0.5% (w/v) casein in Tris-HCl (pH 9.0) at 60◦C for 10 min. The 1mL reaction was terminated by adding 0.5mL of 10% trichloroacetic acid. It was left for 15 min and then centrifuged at 14000 g for 10 min. One unit of enzyme activity was defined as the amount of enzyme required to release 1 μg of tyrosine/min under standard conditions [30]
2.9 Optimization and determination of Kinetic Parameters of Protease Enzyme
2.9.1 Effect of pH on protease activity
The effect of pH on enzyme activity was carried out by incubating the reaction mixture at 40oC over a pH range of 4-9. This was achieved using various buffers at different pH ranges; 0.05M sodium citrate buffer (pH 4-6) and 0.05M Tris-HCl (pH 7-9). Then the enzyme activity was determined by the standard enzyme assay.
2.9.2 Effect of temperature on protease activity
The effect of temperature on enzyme stability was carried out by incubating the reaction mixture over a varied temperature of 30 to 80oC at a predetermined pH. Then the enzyme activity was determined by the standard enzyme assay.
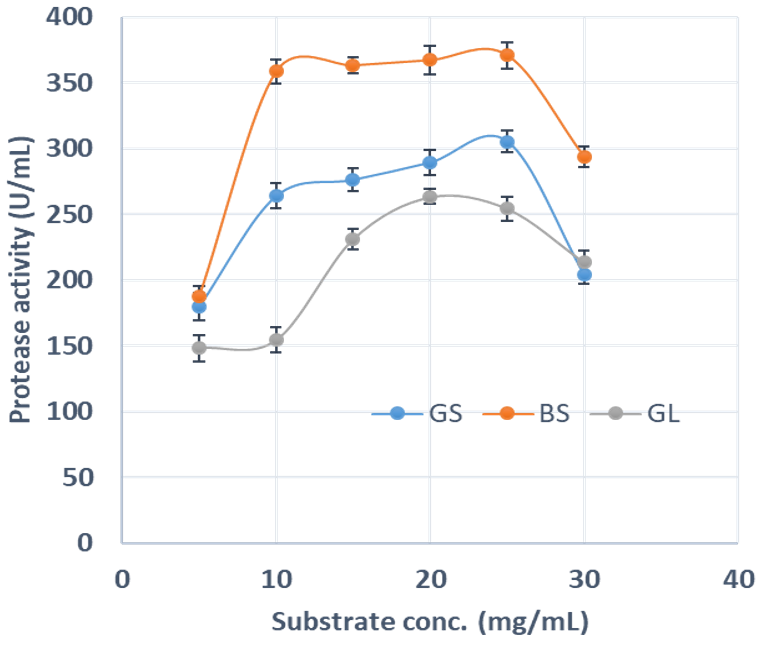
2.9.3 Effect of substrate concentration on protease activity
Effect of substrate concentration on protease activity was determined in reaction mixtures containing varied concentrations of casein solution (mg/ml); 2.5, 5.0, 10.0, 25.0, 30.0, 35.5. Michaelis-Menten constant (Km) and maximum velocity (Vmax) of protease were calculated from the plotted graph of 1/Vo against [1/S.
2.10 Data Analysis
The analysis was conducted in triplicate. Statistical evaluation of data was performed by using Statistical Package for Social Sciences (SPSS). The values reported are means ± SEM. One-way analysis of variance followed by Duncan post hoc test for multiple comparisons was conducted. Values with a p< 0.05 (confidence level = 95%) are consider significant
3.0 Results and Discussion
3.1 Proximate composition of groundnut shell and Bean chaff
The Proximate composition of groundnut shell and Bean chaff are shown in table 1. The Groundnut shell and Bean chaff have low moisture contents of 8.05±0.45 % and 7.53±0.14% respectively. Carbohydrate is the most abundant proximate contents having percentage compositions of 36.14±3.46% and 36.69±2.90% for groundnut shell and Bean chaff respectively. The protein contents were 6.10±0.14% and 8.75±0.13%, while the ash contents were 15.87±1.33% and 6.74±0.46% for groundnut shell and Bean chaff respectively. The protein content of and bean coat is comparably with 6.75 reported for bean coat in previous study [31], and 4.32% obtained for mango peel [32], but higher than 0.9% for Banana peel, and lower than 11.74% reported for plantain bract [33]. The high carbohydrate content reported in this study is an indication that this by-product could serve as a good source of energy for industrial application, and also for both livestock and human being. Similar high level of carbohydrate has been reported for 57.92% for mango peel [32], 51.1% cassava peel [34] and 48.18% for banana peel.
Table 1. Proximate composition of groundnut shell and Bean chaff
| Agro-wastes | Groundnut shell | Bean chaff |
| Moisture content (%) | 8.05±0.45 | 7.53±0.14 |
| Ash content (%) | 15.87±1.33 | 6.74±0.46 |
| Fat (%) | 11.94±0.46 | 23.84±0.24 |
| Fibre (%) | 21.6±2.14 | 16.4±0.09 |
| Protein (%) | 6.10±0.14 | 8.75±0.13 |
| Carbohydrates (%) | 36.14±3.46 | 36.69±2.90 |
Values are mean ± standard deviation of three (3) independent determinations
3.2 Identification of Bacillus licheniformis.as efficient protease producing microorganism under the carbon source of groundnut shell and Bean chaff
A total of five microorganisms were isolated from the domestic dumpsite and screened for protease production on a skimmed milk agar plate. Among the isolates gotten from the domestic dumpsite, isolate A was observed to possess the highest proteolytic index and thus further screened for protease production by means of submerged fermentation on two different agro-industrial wastes and glucose as carbon sources. The selected isolate was subjected to a series of biochemical tests and was identified as Bacillus licheniformis.
The Bacillus licheniformis (Table 2), shows the highest proteolytic activity (due to its ability to hydrolyze skimmed milk) among the other isolated tested for protease production by submerged fermentation using groundnut shell, Bean chaff and glucose as carbon sources. The ability of the isolate to hydrolyze skimmed milk implies that the isolate was able to produce protease(s) which are known to degrade proteins. The presence of the isolate in the domestic dumpsite from which it was isolated may be as a result of the presence of protein-rich kitchen wastes dumped at the site [35]. Therefore, from these findings, it is safe to say that the domestic dumpsite harbours a number of proteolytic bacteria that can be used in many industries.
Table 2: Biochemical tests for the identification of the selected isolate
| Test | Inference |
| Gram reaction | + |
| Shape | Rod |
| Catalase | + |
| H2S | NA |
| Starch hydrolysis | + |
| Glucose | + |
| Mannitol salt agar | + |
| Citrate test | + |
| Urease test | + |
| Methyl Red | NA |
| Vogue Proska | + |
| Indole | _ |
| Lactose | + |
| Slant | NA |
| Butt | NA |
| Isolate | Bacillus licheniformis |
3.3 Effect of change in pH, Temperature, and substrate concentrations on proteases produced by Bacillus licheniformis
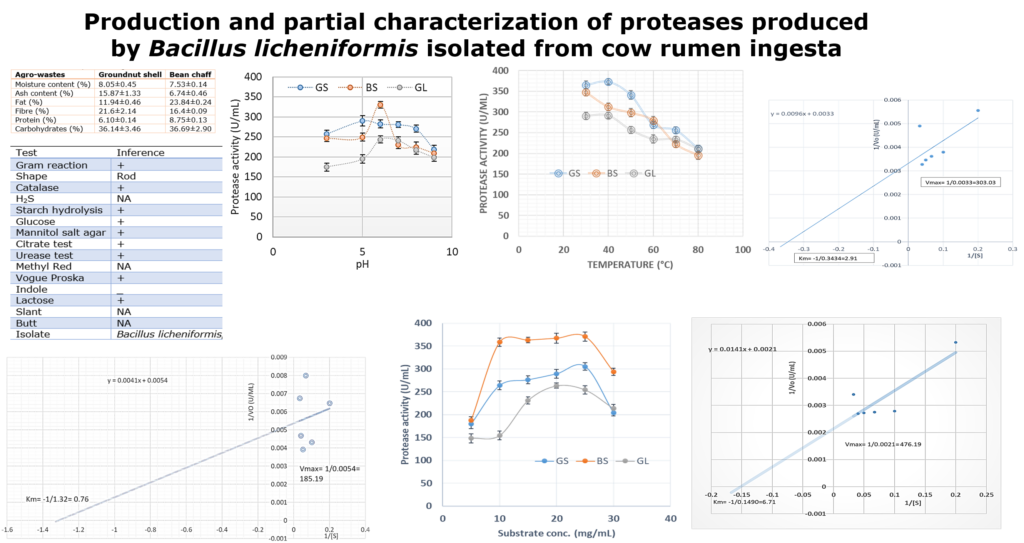
Agro-industrial wastes support the growth of microorganisms by providing them with the nutrients required for their growth. These microorganisms are of industrial importance because they are used for the production of a number of industrial products including enzymes [36]. Hence, the ability of B. lichenformis to grow on groundnut shell and Bean chaff just like it grew on glucose (standard) means that the agro-industrial wastes provided the bacterium with the required nutrients for growth and thus able to support protease production. This invariably implies that the agro-industrial wastes can be used in place of glucose for protease production. In order for a protease to be used in numerous industrial processes, such enzymes must be active and stable under certain pH and temperature conditions. The optimum pH of the proteases produced by B. lichenformis grown on Bean chaff and glucose was observed at pH 6, while that of groundnut shell was recorded at pH 5 (Figure 1). The optimum temperature was observed at 40 °C for proteases produced with groundnut shell and glucose, while that of Bean chaff was observed at 30 °C (Figure 2). A decrease in enzyme activity observed beyond optimum pH and temperatures may be a result of enzyme denaturation. The difference in the properties of proteases produced with agro-industrial wastes and glucose could be a result of differences in nutritional compositions of the carbon sources (Figure 3). However, under optimum pH and temperature conditions, the activities of proteases produced with agro-industrial wastes were higher than that of glucose
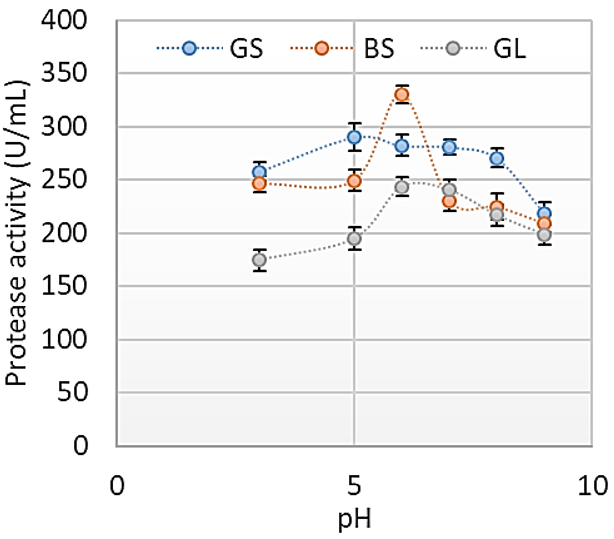
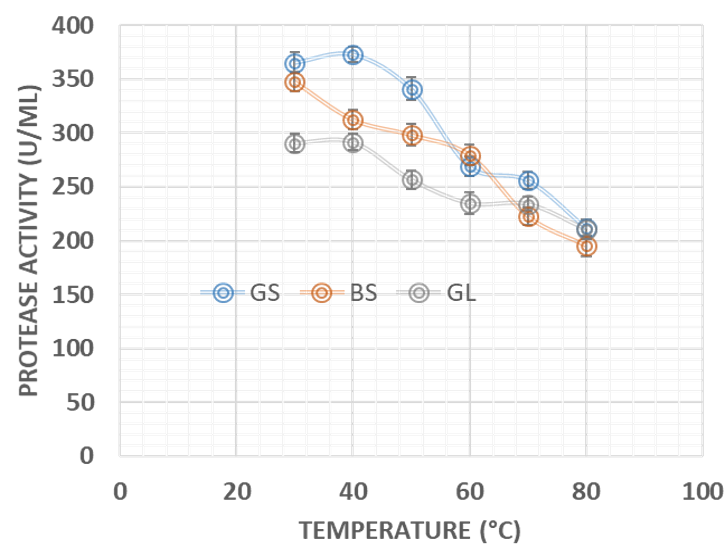
Figure 2: Effect of change in temperature on the activities of proteases produced by Bacillus licheniformis grown on groundnut shell, Bean chaff and glucose as carbon sources
3.4 Kinetics properties of proteases produced by Bacillus licheniformis
The Vmax of the proteases produced by B. lichenformis grown on groundnut shell (303.03 U/mL, Figure 4) and Bean chaff (476.19 U/mL, Figure 5) were significantly higher (p<0.05) than that of glucose (185.19 U/mL), while the Km of glucose (0.76 mg/mL) (Figure 6) was observed to be significantly lower (p<0.05) than that of groundnut shell (2.91 mg/mL) and Bean chaff (6.71 mg/mL) (Figures 4 and 5).
The Km value indicates the affinity of enzymes for a particular substrate and a low Km implies strong affinity. Therefore, the lower Km of proteases produced by B. lichenformis grown on glucose implies that the bacterium preferred glucose more than the agro-industrial wastes as a carbon source. However, after glucose, the enzyme preferred groundnut shell as a carbon source than the Bean chaff. This may be as a result of the higher nutritional content of the groundnut shells (Table 1). Aguilar et al., [37] also reported a low Km value (1.60 mg/mL) for protease produced by B. lichenformis grown on agro-industrial wastes.
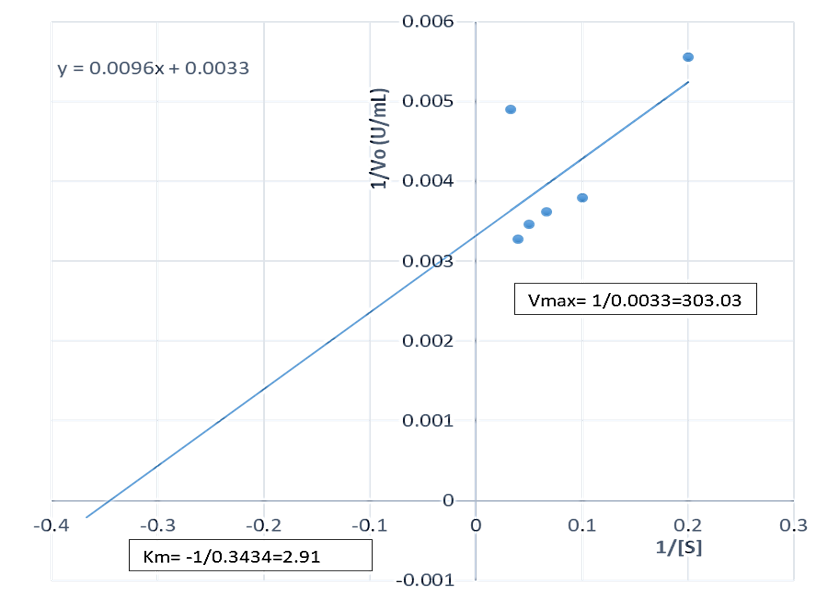
Figure 4: Double reciprocal plot of protease produced by Bacillus licheniformis grown on groundnut shell
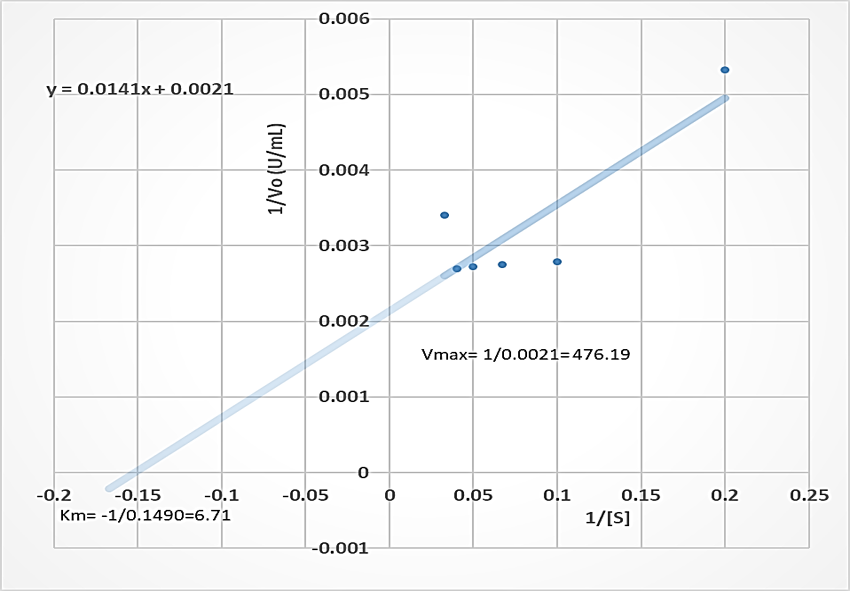
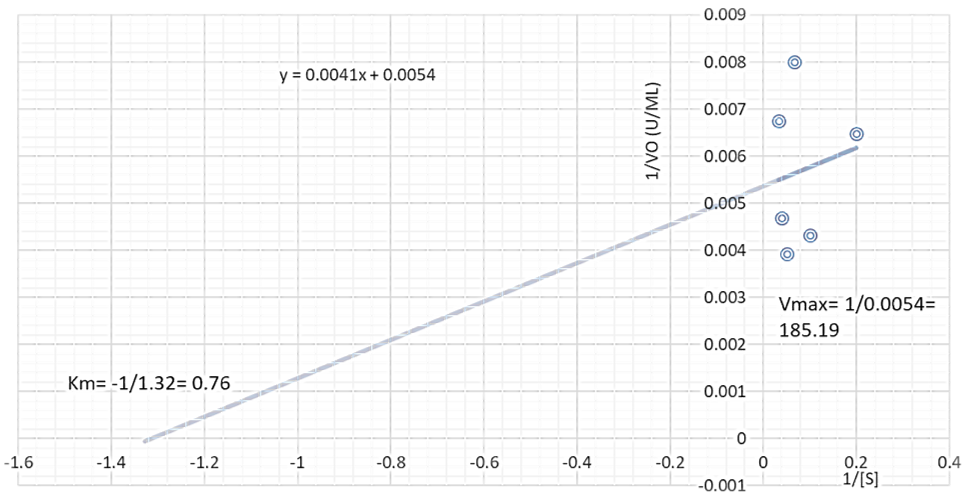
Figure 6: Double reciprocal plot of protease produced by Bacillus licheniformis grown on glucose
4.0 Conclusion
From the results obtained in this study, it could be concluded the screened agro-industrial wastes served as better carbon sources for B. lichenformis and could be used in place of glucose. However, groundnut shell showed a more promising effect as a carbon source for protease production than Bean chaff due to thermal stability and lower Km of protease produced with it than of Bean chaff. The presence of B. lichenformis reveals that domestic dumpsite could be an important source of proteolytic bacteria with numerous industrial applications.
Conflict of interest: The authors declare no conflict of interest.
Authors’ Contributions: This work was conducted in collaboration of all authors. All authors read and approved the final version of the manuscript.
Funding: This study received no external funding
Authors’ contributions: All authors participate in the execution of this project. All authors read and approved the final manuscript.
Reference
5. Ward, O. Proteases. Comprehensive biotechnology 2011, 571.
7. Loehr, R. Agricultural waste management: problems, processes, and approaches; Elsevier: 2012.
15. Duc, P.A.; Dharanipriya, P.; Velmurugan, B.K.; Shanmugavadivu, M. Groundnut shell -a beneficial bio-waste. Biocatalysis and Agricultural Biotechnology 2019, 20, 101206, doi:https://doi.org/10.1016/j.bcab.2019.101206.
16. Gordillo, M. El garbanzo: Una alternativa para el secano. Agroguías Mundi-Prensa 1989.
19. Hameed, B.H.; El-Khaiary, M.I. Sorption kinetics and isotherm studies of a cationic dye using agricultural waste: Broad bean peels. Journal of Hazardous Materials 2008, 154, 639-648, doi:https://doi.org/10.1016/j.jhazmat.2007.10.081.